��Ŀ����
�������س��õ�NaNO2����ۺ�ʳ�����ƣ�������ζ������ʹ����ʳ�ж�����֪NaNO2�ܷ������·�Ӧ��
��1����ƽ���������ת�Ƶķ������Ŀ��
��2��������Ӧ���������� ������1 mol�Ļ�ԭ������������Ӧ��ת�Ƶ��ӵĵ���Ŀ�� ��
��3������������Ӧ��������ֽ�������г��������ʽ���ʵ�飬�Լ���NaNO2��NaCl����ѡ�õ������У���ˮ �ڵ⻯�ص�����ֽ �۵��� �ܰ� ��ʳ�ף�����ʵ�飬���м�����ʵ��� ������ţ���
| A���ۢ� | B���٢ڢ� |
| C���٢ڢ� | D���٢ڢۢ� |
��5�������ռ�����Ṥҵβ�����Ի�ø���ƷNaNO2�����Ϊ����������aL bmol/L���ռ�����Ṥҵβ���������Ի��NaNO2�����ʵ���Ϊ mol��
��1�� 2NaNO2 + 4HI ��2NO + I2 +2 NaI +2 H2O��3�֣�
��2��NaNO2��1�֣���6��02��1023��NA��1�֣���3��C ��2�֣�
��4��NH4Cl + NaNO2 ��NaCl + N2��+2H2O ��2�֣�NH4Cl��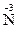 ��1�֣���5��ab ��2�֣�
��1�֣���5��ab ��2�֣�
����

��9�֣��������س��õ�NaNO2����ۺ�ʳ�����ƣ�������ζ������ʹ����ʳ�ж�����֪NaNO2�ܷ������·�Ӧ��

��1����ƽ���������ת�Ƶķ������Ŀ��
��2��������Ӧ���������� ������1 mol�Ļ�ԭ������������Ӧ��ת�Ƶ��ӵĵ���Ŀ�� ��
��3������������Ӧ��������ֽ�������г��������ʽ���ʵ�飬�Լ���NaNO2��NaCl����ѡ�õ������У��� ˮ �� �⻯�ص�����ֽ  �� ���� �� �� �� ʳ�ף�����ʵ�飬���м�����ʵ��� ������ţ���
�� ���� �� �� �� ʳ�ף�����ʵ�飬���м�����ʵ��� ������ţ���
| A���ۢ� | B���٢ڢ� |
| C���٢ڢ� | D���٢ڢۢ� |
��5�������ռ�����Ṥҵβ�����Ի�ø���ƷNaNO2�����Ϊ����������a L b mol/L���ռ�����Ṥҵβ���������Ի��NaNO2�����ʵ���Ϊ mol��

