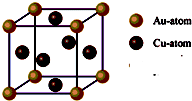
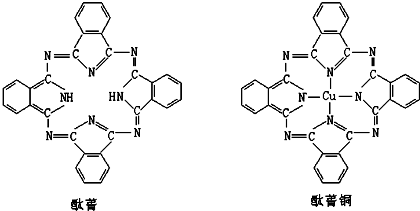
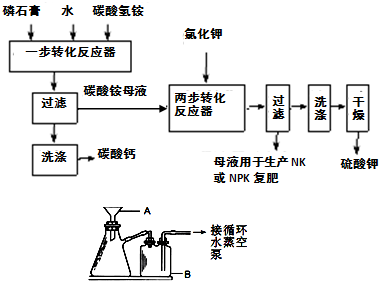
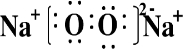��Ŀ����
��������������Ҫ�Ľ��������ǵĵ��ʼ����������Ÿ��Ե����ʡ�(1)��һ���¶��£�������������һ����̼�������з�Ӧ��
Fe2O3(s)+3CO(g)![]() 2Fe(s)+3CO2(g)
2Fe(s)+3CO2(g)
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪ��K=______________________��
�ڸ��¶��£���
(2)����������Ӧ��ij��������й���������˵���÷�Ӧ�Ѵﵽƽ��״̬��
��_______________________����_______________________��
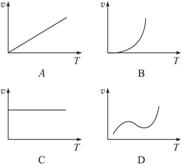
(3)ijЩ�����������ĩ��Al����þ������ȼ�¿��Է������ȷ�Ӧ�����з�Ӧ����(v)���¶�(T)�Ĺ�ϵʾ��ͼ�������ȷ�Ӧ��ӽ�����_______________________��
(4)д������������ˮ�з�����ʽ����ĵ��뷽��ʽ��______________________________________________����ʹ������ϵ��Al3+Ũ�����ӣ��ɼ����������_______________________��
(1)![]() 0.015 mol��(L��min)-1
0.015 mol��(L��min)-1
��2����CO����CO2������������������������� ��CO����CO2�����������ٸı�
��3��b
��4��Al(OH)3![]() H++
H++![]() +H2O ����
+H2O ����
����:��1��Fe2O3(s)��3CO(g)![]() 2Fe(s)��3CO2(g)
2Fe(s)��3CO2(g)
3 mol 2��
0.3 mol
![]()
��3�������¶����ߣ���Ӧ���������жϿ�֪��ͼ��b��ȷ��
��4��Al(OH)3�����������������ʽ��������ӷ���ʽΪAl(OH)3![]() H����
H����![]() ��H2O����ʽ��������ӷ���ʽΪAl(OH)3====Al3����3OH-����ʹAl3��Ũ�����ɼ������ᡢ������Խ���OH-Ũ�ȣ�ʹƽ�����ʽ����ķ����ƶ���
��H2O����ʽ��������ӷ���ʽΪAl(OH)3====Al3����3OH-����ʹAl3��Ũ�����ɼ������ᡢ������Խ���OH-Ũ�ȣ�ʹƽ�����ʽ����ķ����ƶ���

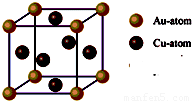
 ����С����ͬ������ϵ�д�
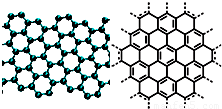
����С����ͬ������ϵ�д�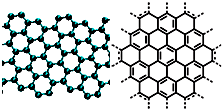 A����������ѧ����־������2009��ʮ���ѧͻ��֮һ��ʯīϩ���о���Ӧ�÷����ͻ�ƣ�ʯīϩ����ԭ�Ӽ��ĺ�ȡ�����ĵ�ѧ���ܡ���ɫ�Ļ�ѧ�ȶ��Ժ�����ѧ�ȶ��ԣ��Ʊ�ʯīϩ������ʯī���뷨����ѧ����������ȣ�ʯīϩ�����ģ�ͼ����ӽṹʾ��ͼ���ң�
A����������ѧ����־������2009��ʮ���ѧͻ��֮һ��ʯīϩ���о���Ӧ�÷����ͻ�ƣ�ʯīϩ����ԭ�Ӽ��ĺ�ȡ�����ĵ�ѧ���ܡ���ɫ�Ļ�ѧ�ȶ��Ժ�����ѧ�ȶ��ԣ��Ʊ�ʯīϩ������ʯī���뷨����ѧ����������ȣ�ʯīϩ�����ģ�ͼ����ӽṹʾ��ͼ���ң�