��Ŀ����
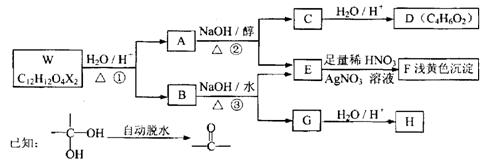
����ͼ��ʾ����A�����ɷ���һϵ�л�ѧ��Ӧ�����л�����B�����������������ʱ��һ�����ֻ�����֣�F���Ȼ�����Һ����ɫ��GΪ�߷��ӻ����
��֪��
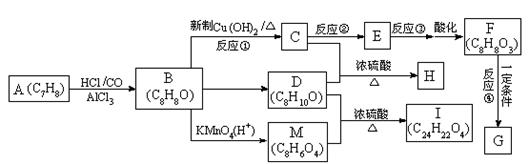
�����������Ϣ��գ�
��1��д����Ӧ���ͣ���Ӧ��_____________________����Ӧ��______________________��
��2������Ӧ�۵�����Ϊ����������Һ�����ȣ���Ӧ�ڵ���������Ϊ________��ѡ��𰸱�ţ���
��3��H�Ľṹ��ʽΪ_______________________________________________��
��4����C��Ϊͬ���칹���Ҿ���������ķ�����Ļ����ﹲ��_____�֣����б����ϵ�һȡ���������ֵ����ʵĽṹ��ʽΪ_____________________________________��
��5��д�����з�Ӧ�Ļ�ѧ����ʽ��
F��G��________________________________________________________________��
D + M ��I��____________________________________________________________��
��֪��
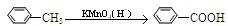

�����������Ϣ��գ�
��1��д����Ӧ���ͣ���Ӧ��_____________________����Ӧ��______________________��
��2������Ӧ�۵�����Ϊ����������Һ�����ȣ���Ӧ�ڵ���������Ϊ________��ѡ��𰸱�ţ���
| A����ˮ������ | B��Һ�塢���� |
| C�������ᡢ���� | D������������ |
��4����C��Ϊͬ���칹���Ҿ���������ķ�����Ļ����ﹲ��_____�֣����б����ϵ�һȡ���������ֵ����ʵĽṹ��ʽΪ_____________________________________��
��5��д�����з�Ӧ�Ļ�ѧ����ʽ��
F��G��________________________________________________________________��
D + M ��I��____________________________________________________________��
��1����2�֣�������Ӧ�����۷�Ӧ��
��2����1�֣�D
��3����2�֣�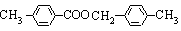 ��
��
��4����3�֣�6�� ��
��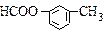 ��
��
��5����4�֣�F��G��n

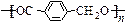 + n H2O��
+ n H2O��
D + M ��I��2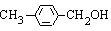 +
+
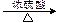
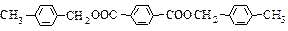 + 2H2O
+ 2H2O
��2����1�֣�D
��3����2�֣�
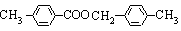 ��
����4����3�֣�6��
 ��
��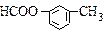 ��
����5����4�֣�F��G��n


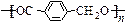 + n H2O��
+ n H2O��D + M ��I��2
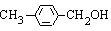 +
+
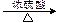
 + 2H2O
+ 2H2O��

��ϰ��ϵ�д�
 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д�
�����Ŀ
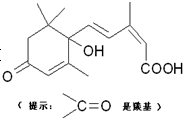
 ��B�Ľṹ��ʽ ��
��B�Ľṹ��ʽ ��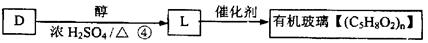
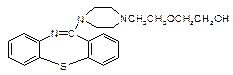 )2��
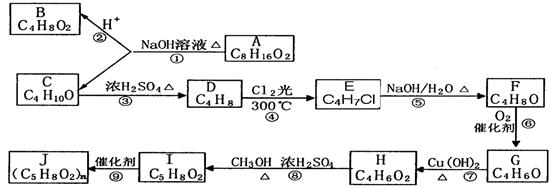
)2��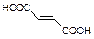 �����ĺϳ�·�����£�
�����ĺϳ�·�����£�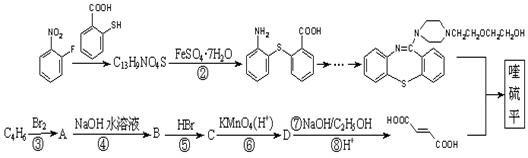
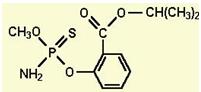
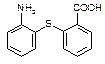 �г����Ѽ�����S�����⣬�����еĹ���������Ϊ ��
�г����Ѽ�����S�����⣬�����еĹ���������Ϊ ��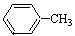 Ϊԭ�Ϻϳ�
Ϊԭ�Ϻϳ�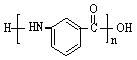 ���úϳ�·������ͼ��ʾ����
���úϳ�·������ͼ��ʾ����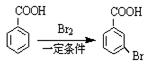 ���������
���������
 ����ͼ��ʾ��ͼ��
����ͼ��ʾ��ͼ�� ������֮�����ߴ�����ѧ��������˫���ȡ���
������֮�����ߴ�����ѧ��������˫���ȡ���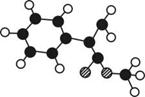
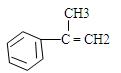 �������ϳ�A����ϳ�·�����£�
�������ϳ�A����ϳ�·�����£�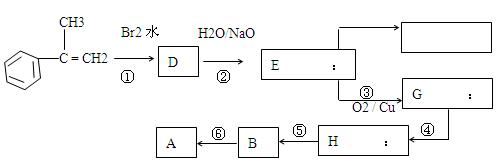
 Ӧ����ʽ��ע����Ҫ�ķ�Ӧ������
Ӧ����ʽ��ע����Ҫ�ķ�Ӧ������