��Ŀ����
�������糧�ͷų�������������(NOx��������������������ɻ�����Ⱦ����ȼú���������ѳ���������ʵ����ɫ��������̼���š��������õ�Ŀ�ġ�
��1�����������ü������ԭNOx��CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g����H1=-574kJ/mol CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g����H2=-1160 kJ/mol
����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ______��
��2����̼��
��CO2ת��Ϊ�״����Ȼ�ѧ����ʽΪ��CO2��g��+3H2��g�� CH3OH��g��+H2O��g����H3
CH3OH��g��+H2O��g����H3
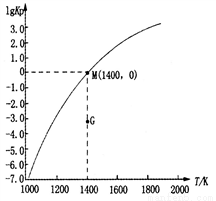
��ȡ��ݵ����CO2��H2�Ļ�����壨���ʵ���֮�Ⱦ�Ϊ1��3�����ֱ�����¶Ȳ�ͬ���ݻ���ͬ�ĺ����ܱ������У�����������Ӧ����Ӧ��ͬʱ���ü״�����������գ�CH3OH���뷴Ӧ�¶�T�Ĺ�ϵ���ߣ�����ͼ����������CO2ת��Ϊ�״���Ӧ�ġ�H3______0�����������������=������

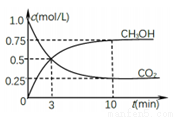
����һ���º����ܱ������г���0.5molCO2��1.5molH2������������Ӧ�����CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ����ش�0��10min�ڣ�������ƽ����Ӧ����_____����10min�������£���ʹc(CH3OH)/c(CO2)_____(��������С���������䡱)���ж�������_________��������������ٳ���1molCO2��3molH2,�ٴδﵽƽ��ʱ����CH3OH(g)�����������_________(���������С���������䡱)��
��3������ij���������н���������������һ�����İ�����������Ӧ����������狀�����淋Ļ������Ϊ����Ʒ���ʡ��������е�SO2��NO2�����ʵ���֮��Ϊ1��1����÷�Ӧ�Ļ�ѧ����ʽΪ___________������0.1mol/L���������Һ�м���������0.09mol/L��ˮ��������pH=8.2����Һ�������Һ����Ũ����С�����˳��Ϊ________________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�

 CH2Cl2+H2 ȡ����Ӧ
CH2Cl2+H2 ȡ����Ӧ CH3CHBr2 �ӳɷ�Ӧ
CH3CHBr2 �ӳɷ�Ӧ 2CH3COOH �ӳɷ�Ӧ
2CH3COOH �ӳɷ�Ӧ CH3COOH+C2H5OH ȡ����Ӧ
CH3COOH+C2H5OH ȡ����Ӧ H2(g)+I2(g)����HI��Ũ����0.1 mol • L-1����0.07 mol • L-1ʱ��Ҫ15s����HI��Ũ����0.07 mol • L-1����0.05 mol • L-1ʱ������ʱ��Ϊ
H2(g)+I2(g)����HI��Ũ����0.1 mol • L-1����0.07 mol • L-1ʱ��Ҫ15s����HI��Ũ����0.07 mol • L-1����0.05 mol • L-1ʱ������ʱ��Ϊ C2H2(g)+3H2(g)��H��ʵ���ø÷�Ӧ��Kp(��ƽ���ѹ����Ũ�ȼ����ƽ�ⳣ������ѹ=��ѹ�����ʵ�������)���¶ȵĹ�ϵ��ͼ��ʾ��
C2H2(g)+3H2(g)��H��ʵ���ø÷�Ӧ��Kp(��ƽ���ѹ����Ũ�ȼ����ƽ�ⳣ������ѹ=��ѹ�����ʵ�������)���¶ȵĹ�ϵ��ͼ��ʾ��