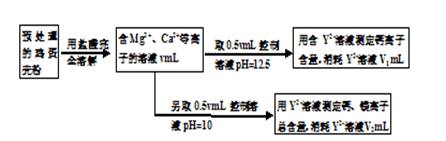
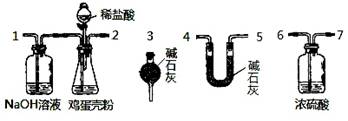
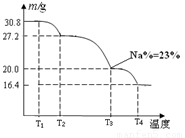
��Ŀ����
��֪��Ӧ��Mg(OH)2��MgO(s)+H2O(g)��2Al(OH)3(s)��Al2O3(s)+ H2O(g)��Ϊ���ȷ�Ӧ1mol Mg(OH)2�ֽ�����81.5kJ��1mol Al(OH)3�ֽ�����87.7kJ�����У�����þ�۵�2852�棻�������۵�2050��
��1��Mg(OH)2��Al(OH)3����ȼ���õ���Ҫԭ����________________________��
��2��������Mg(OH)2��Al(OH)3��ȣ���ȼЧ���Ϻõ���__________________��ԭ����______________��
��3��������ȼ����Ҫ�����ࣺ
A.±ϵ�����������飨�۵㣰�棬�е㣺243.5�棩��
B.��ϵ�����������������۵�48.5�棬�е�370�棩��
C.���࣬��Ҫ��Mg(OH)2��Al(OH)3��
�ӻ����ĽǶȿ��ǣ�Ӧ��ʱ���������ȼ����________________(�����)��
��1��Mg(OH)2��Al(OH)3����ȼ���õ���Ҫԭ����________________________��
��2��������Mg(OH)2��Al(OH)3��ȣ���ȼЧ���Ϻõ���__________________��ԭ����______________��
��3��������ȼ����Ҫ�����ࣺ
A.±ϵ�����������飨�۵㣰�棬�е㣺243.5�棩��
B.��ϵ�����������������۵�48.5�棬�е�370�棩��
C.���࣬��Ҫ��Mg(OH)2��Al(OH)3��
�ӻ����ĽǶȿ��ǣ�Ӧ��ʱ���������ȼ����________________(�����)��
��1��Mg(OH)2��Al(OH)3���ȷֽ�ʱ���մ������ȣ�ʹ�����¶��½���ͬʱ���ɵ����¡��ȶ��Ժõ�
MgO��Al2O3�����ڿ�ȼ����棬��ȼЧ������
��2��Mg(OH)2����������Mg(OH)2��Al(OH)3���ȶ�
��3��C
MgO��Al2O3�����ڿ�ȼ����棬��ȼЧ������
��2��Mg(OH)2����������Mg(OH)2��Al(OH)3���ȶ�
��3��C

��ϰ��ϵ�д�
 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
�����Ŀ
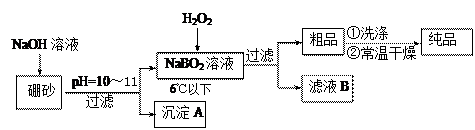
 ���н�����NaBO2 �����Ʒ���Ƶ��������� �� ���� �����ߡ��������͡����䡱����[��Դ:ѧ����ZXXK]
���н�����NaBO2 �����Ʒ���Ƶ��������� �� ���� �����ߡ��������͡����䡱����[��Դ:ѧ����ZXXK]