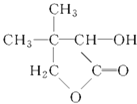
��Ŀ����
����Ŀ����֪A��B��C��D��ԭ������������������ֶ���������Ԫ�أ�A������������������������Bԭ�ӵļ۵����Ų�Ϊnsnnpn��D�ǵؿ��к�������Ԫ�ء�E�ǵ�������p����Ԫ���������ֻ��2�ԳɶԵ��ӣ�F��29��Ԫ�ء�
��1��B��C��D��Ԫ�ص�һ�������ɴ�С��˳��Ϊ ����Ԫ�ط��ű�ʾ��
��2��BD32-����ԭ���ӻ����������Ϊ________�ӻ���CA4+�Ŀռ乹��Ϊ______________��
��3����̬Eԭ�ӵļ۵����Ų�ͼ______________________________��
��4��1mol BC���к��Цм�����ĿΪ______________��
��5���Ƚ�D��EԪ������⻯��ķе�ߵͣ� ���û�ѧʽ��ʾ����
��6��C��F��Ԫ���γɵ�ij������ľ����ṹ��ͼ��ʾ������ΪCԭ�ӡ���û�����Ļ�ѧʽ�� ��Cԭ�ӵ���λ���� ��������Cԭ�Ӻ�Fԭ�Ӽ�ľ���Ϊa cm������٤������ΪNA����þ�����ܶ�Ϊ________________g��cm3���ú�a��NA�ķ��ű�ʾ����

���𰸡���1��N��O��C
��2��sp2 ��������
��3��![]()
��4��2NA
��5��H2O��H2Se
��6��Cu3N 6 103/4a3NA
��������
������������������Ϣ�� A��B��C��D��ԭ������������������ֶ���������Ԫ�أ�A������������������������Bԭ�ӵļ۵����Ų�Ϊnsnnpn��D�ǵؿ��к�������Ԫ�أ���AΪH��BΪ̼��DΪ������CΪ��Ԫ�أ�E�ǵ������ڵ�p��Ԫ���������ֻ��2�ԳɶԵ��ӣ�EΪSe��FԪ�ص�ԭ������Ϊ29��FΪͭ����
��1��ͬ�����������ҵ�һ��������С����NԪ�ص�2p������Ӵ��ڰ����״̬���ȶ���ǿ����һ�����ܴ�����Ԫ�أ���B��C��D��Ԫ�ص�һ�������ɴ�С��˳��ΪN��O��C��
��2��CO32-����ԭ��̼ԭ�ӵļ۲���Ӷ�����3+![]() ��������ӻ����������Ϊsp2�ӻ���NH4+�Ŀռ乹��Ϊ�������塣
��������ӻ����������Ϊsp2�ӻ���NH4+�Ŀռ乹��Ϊ�������塣
��3��Se��ԭ��������34������ݺ�������Ų����ɿ�֪��̬Seԭ�ӵļ۵����Ų�ͼΪ![]() ��
��
��4��CN-������C��N��ΪC��N��1mol CN���к��Цм�����ĿΪ2NA��
��5��ˮ���Ӽ������������⻯��е�ΪH2O��H2Se��
��6�����ݾ����Ľṹ��֪������Cu���������![]() ��������N�������Ϊ
��������N�������Ϊ![]() ������������Ļ�ѧʽCu3N�������N3-��1/8����Ӧ������������3��Cu+��1/4������N3-��Cu+=1/8��3/4=1��6������λ����6�������������V=��2a��3cm3������Ϊ206/NA�����ܶ�Ϊ103/4a3NA��
������������Ļ�ѧʽCu3N�������N3-��1/8����Ӧ������������3��Cu+��1/4������N3-��Cu+=1/8��3/4=1��6������λ����6�������������V=��2a��3cm3������Ϊ206/NA�����ܶ�Ϊ103/4a3NA��