��Ŀ����
ij�¶�����ˮ�д������µ���ƽ�⣺D2O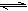 D++OD-��KW��D2O��=1.0��10-12mol2?L-2����pD=-lg��D+�ݣ����ڸ��¶��£�
D++OD-��KW��D2O��=1.0��10-12mol2?L-2����pD=-lg��D+�ݣ����ڸ��¶��£���1��0.1molNaOD������ˮ�γ�1L��Һ����pD=_____��
��2����pD=4��DCl����ˮ��Һ������ˮϡ��100������pOD=________��
��3����30mL0.5mol?L-1NaOD����ˮ��Һ�м���20mL0.5mol?L-1DCl����ˮ��Һ��������Һ��pD=______��
��4��pD=10��NaOD��ˮ��Һ�У�����ˮ������ģ�OD-��=_______mol?L-1��
��1��11��������
��2��6.3����������
��3��11��������
��4��1.0��10-10
�����������

��ϰ��ϵ�д�
�����Ŀ




 D++OD-��Kw��D2O��=1.0��10-12����pD=-lg{c(D+)},����¶����йط�������ȷ���ǣ�
��
D++OD-��Kw��D2O��=1.0��10-12����pD=-lg{c(D+)},����¶����йط�������ȷ���ǣ�
�� ��ƽ�⣺D2O
��ƽ�⣺D2O  D++OD-��Kw��D2O��=1.0��10-12����pD=-lg{c(D+)},����¶����йط�������ȷ���ǣ� ��
D++OD-��Kw��D2O��=1.0��10-12����pD=-lg{c(D+)},����¶����йط�������ȷ���ǣ� ��