��Ŀ����
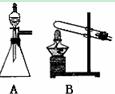
��ͼ��ʵ������ȡ��������ֳ�������װ�ã�
(1)ʵ������ʯ��ʯ��ϡ���ᷴӦ��ȡ������̼��Ӧѡ�õķ���װ
���� ����ѡ��A"��B"����
��Ӧ�����ӷ���ʽΪ
(2)������غͶ�����������ȡ������Ӧѡ�õķ���װ����________����ѡ��A"��B"����
��ѧ��Ӧ����ʽΪ����������������������������������������������
(3)����Aװ����ȡ��������ѡ�Լ�Ϊ ��
��Ӧ�Ļ�ѧ����ʽΪ
(4)��(2)(3)���ַ�����ȡ����ʱ����������ͬ��������������(2)(3)����Ӧת�Ƶĵ�����֮��Ϊ ��
(1) A CaCO3 + 2H+ =Ca2++CO2��+2H2O
(2) B
(3) H2O2 ��MnO2 ��
(4) 2:1
����:��

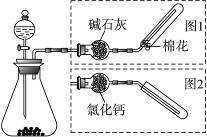
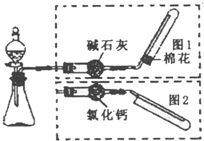 ��ͼ��ʵ������ȡ�����װ�ã����з���װ����ͬ������ͼ���װ�������ף��ֱ���ͼ1��ͼ2��ʾ������ѡ������ȷ���� ��ͼ��ʵ������ȡ�����װ�ã����з���װ����ͬ������ͼ���װ�������ף��ֱ���ͼ1��ͼ2��ʾ������ѡ������ȷ����
|
��ͼ��ʵ������ȡ�����װ�ã����з���װ����ͬ������ͼ���װ�������ף��ֱ���ͼ1��ͼ2��ʾ������ѡ������ȷ����
| ����װ���е�ҩƷ | ����ͼ���װ�� | |
| A | ��ʯ��ˮ | ͼ2 |
| B | ����ʯ��ϡ���� | ͼ1 |
| C | ͭ��ϡ���� | ͼ2 |
| D | �����ƺ�Ũ��ˮ | ͼ1 |