��Ŀ����
(12��) ʵ��������������(��Ҫ�ɷ���Al2O3��������SiO2��Fe2O3����)Ϊԭ����ȡAl2(SO4)3�����������[NH4Al(SO4)2��12H2O]�Ĺ����������£�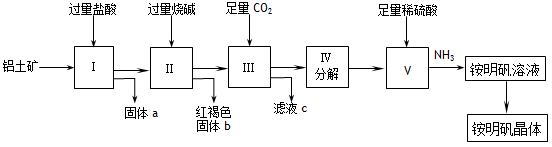
�Իش��������⣺
�Ź���a�Ļ�ѧʽΪ_____��III��ͨ�����CO2��������ӷ���ʽΪ ��
����V��ȡ�������Һ�Ļ�ѧ����ʽΪ__________________________�����������Һ�л������������ʵ���������Ϊ����������ƣ� ����ȴ�ᾧ������ϴ�ӡ�
����1000kg��������36%��������Ϊԭ����ȡAl2(SO4)3����������������98%�����ᣨ�ܶ�1.84g/cm3��___________L������һλС������
����ͬʱ��ȡ���������������ͨ����������������������ֲ�Ʒ�IJ���������ʹ�Ƶõ�������������������ʵ���֮��Ϊ1:1����Ͷ��ʱ��������Al2O3��H2SO4�����ʵ���֮��Ϊ___________��
��12�֣�
��SiO2��2�֣� AlO2-+CO2+2H2O ==HCO3-+Al(OH)3����2�֣�
��Al2(SO4)3 + H2SO4 + 2NH3 ==2NH4Al(SO4)2��2�֣� ����Ũ����2�֣�
��575.4L��2�֣� ��3:10��2�֣�
����