��Ŀ����
��X��Y��Z��W���ֺ�14�����ӵ����ӣ���ṹ�ص����£�
(1)Aԭ�Ӻ����Xԭ�Ӷ�3�����ӣ�A��ԭ�ӽṹʾ��ͼ�� ����lmolX�������ᄃ���к��й��ۼ���ĿΪ ��
(2)Z���������ɵĻ�����ĵ���ʽΪ ��
(3)14gY��ȫȼ�շų���������141.5kJ��д��Yȼ�յ��Ȼ�ѧ����ʽ ��
(4)���W��Ԫ������������Ӧ��ˮ���������ͼ��ʾת����ϵ����Ϊ�������ʣ���Ӧ�����������������ԣ�

��д����Ԫ�������ڱ��е�λ�� ��
��д�����ڸ�������ˮ��Ӧ�Ļ�ѧ����ʽ ��
�����W��Ԫ�صļ��⻯�K������ˮ����Ҫԭ���� �����⻯����������Թ���һ��ȼ�ϵ�أ��������Һ��KOH���为���ĵ缫��ӦʽΪ ��
| ���Ӵ��� | X | Y | Z | W |
| ԭ�Ӻ��� | ���� | ��ͬԪ�ع��ɵ����� | ͬԪ�ع��ɵ����� | ͬԪ�ع��ɵ����� |
| ���ӵĵ���� | 0 | 0 | ��������� | 0 |
(1)Aԭ�Ӻ����Xԭ�Ӷ�3�����ӣ�A��ԭ�ӽṹʾ��ͼ�� ����lmolX�������ᄃ���к��й��ۼ���ĿΪ ��
(2)Z���������ɵĻ�����ĵ���ʽΪ ��
(3)14gY��ȫȼ�շų���������141.5kJ��д��Yȼ�յ��Ȼ�ѧ����ʽ ��
(4)���W��Ԫ������������Ӧ��ˮ���������ͼ��ʾת����ϵ����Ϊ�������ʣ���Ӧ�����������������ԣ�

��д����Ԫ�������ڱ��е�λ�� ��
��д�����ڸ�������ˮ��Ӧ�Ļ�ѧ����ʽ ��
�����W��Ԫ�صļ��⻯�K������ˮ����Ҫԭ���� �����⻯����������Թ���һ��ȼ�ϵ�أ��������Һ��KOH���为���ĵ缫��ӦʽΪ ��
��1����ԭ�ӽṹʾ��ͼ���ԣ� 4NA��2.408��1024
��2��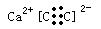
��3��2CO(g)+O2=2CO2(g);��H=-566KJ/mol ����������Ҳ�ɵ÷�
��4���ٵ������ڵڢ��� ��
��NH3��H2O���Ǽ��Է��ӣ���������(��NH3��H2O�����γ����)�� 2NH3-6e+6OH-�TN2+6H2O
��2��
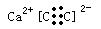
��3��2CO(g)+O2=2CO2(g);��H=-566KJ/mol ����������Ҳ�ɵ÷�
��4���ٵ������ڵڢ��� ��

��NH3��H2O���Ǽ��Է��ӣ���������(��NH3��H2O�����γ����)�� 2NH3-6e+6OH-�TN2+6H2O
������������������֪���������ʷֱ��ǣ�XΪSi,YΪCO,ZΪC22_,ZΪN2.(1)XΪ14��Ԫ��Si����A Ϊ17Ԫ���ȡ���ԭ�ӽṹʾ��ͼ�ԡ���������Ϊԭ�Ӿ��壬ÿ��Siԭ����4��Oԭ��ͨ�����ۼ���ϣ��ʺ�lmolSi�������ᄃ���к��й��ۼ���ĿΪ4NA��2.408��1024.
��2������ZΪC22_,Z���������ɵĻ�����ĵ���ʽ
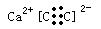
��3��YΪCO,��Ħ������Ϊ28g/mol.n(CO)="0.5" mol���Ȼ�ѧ����ʽΪ��2CO(g)+O2(g)=2CO2(g);��H=-566kJ/mol.����������Ҳ�ɵ÷֡�
��4����������֪��ΪHNO3,�������ᷴӦ���Ҽ���������ٲ�ͬ�����ﲻͬ�Ľ���ֻ��Fe,����Ԫ�����ڱ���λ�ڵ������ڵڢ��壬���ڸ���������ˮ��Ӧ�Ļ�ѧ����ʽ�ǣ�

��NH3��H2O���Ǽ��Է��ӣ�NH3��H2O֮����˷��Ӽ���������⣬�����ڴ������������������á�������������ԭ�����������ܽ���ˮ�������������ɵ�ȼ�ϵ�أ�����Ϊ����������Ϊ�������为���ĵ缫��ӦʽΪ2NH3-6e-+6OH-=N2+6H2O��

��ϰ��ϵ�д�
�����Ŀ

 �� DF�ĵ���ʽΪH��Cl��
�� DF�ĵ���ʽΪH��Cl��
 ��˵�����������
��˵�����������