��Ŀ����
�л�ճ�ϼ���������������һ����Ҫ�IJ��ϣ�ճ�Ϲ���һ����Һ̬��С����ճ�ϼ�����ѧ��Ӧת��Ϊ����ӻ�߷��Ӷ��̻���
��1����502������һ�ֿ�ɽ�������Ҫ�ɷ�Ϊ��-�����ϩ�������� �����ڿ�������ˮ���·����Ӿ۷�Ӧ��Ѹ�ٹ̻�������ճ�Σ���д����502��������ճ�����õĻ�ѧ����ʽ��
�����ڿ�������ˮ���·����Ӿ۷�Ӧ��Ѹ�ٹ̻�������ճ�Σ���д����502��������ճ�����õĻ�ѧ����ʽ��

��2���������� ��Ҳ��һ��ճ�ϼ����ڿ������ȶ������ڸ���������ȱ����ʱ��������˫���Ͽ������ۺ϶��̻�����ҵ���ñ�ϩ���ij��������һ�������·�Ӧ���Ƶ�����ճ�ϼ�����һ��ȡ���̵Ļ�ѧ����ʽΪ
��Ҳ��һ��ճ�ϼ����ڿ������ȶ������ڸ���������ȱ����ʱ��������˫���Ͽ������ۺ϶��̻�����ҵ���ñ�ϩ���ij��������һ�������·�Ӧ���Ƶ�����ճ�ϼ�����һ��ȡ���̵Ļ�ѧ����ʽΪ
��3�����齺�dz��õ�ճ�ϼ�������Ҫ�ɷ�Ϊ������ϩ����CH3COOCH=CH2�������ж���ͬ���칹�壬�� ����֪����
����֪���� �ṹ�����ʲ����ȶ����ڣ���������д���ֺ�-CH=CH-�ṹ����״ͬ���칹��Ľṹ��ʽ��
�ṹ�����ʲ����ȶ����ڣ���������д���ֺ�-CH=CH-�ṹ����״ͬ���칹��Ľṹ��ʽ��
��4����֪��ȩ��Ӧ2CH3OH+HCHO
CH3OCH2OCH3+H2O���Է���������ϩ ��������ͨ��ˮ�������ǻ���ȫ��������ȩ������ˮ���õ�����ԭ�ӻ���ǿ��ճ�ϼ�����ϩ����ȩ����д����ȡ����ϩ����ȩ�Ļ�ѧ����ʽ��
��������ͨ��ˮ�������ǻ���ȫ��������ȩ������ˮ���õ�����ԭ�ӻ���ǿ��ճ�ϼ�����ϩ����ȩ����д����ȡ����ϩ����ȩ�Ļ�ѧ����ʽ��
 ��
��
��1����502������һ�ֿ�ɽ�������Ҫ�ɷ�Ϊ��-�����ϩ��������
 �����ڿ�������ˮ���·����Ӿ۷�Ӧ��Ѹ�ٹ̻�������ճ�Σ���д����502��������ճ�����õĻ�ѧ����ʽ��
�����ڿ�������ˮ���·����Ӿ۷�Ӧ��Ѹ�ٹ̻�������ճ�Σ���д����502��������ճ�����õĻ�ѧ����ʽ��

��2����������
 ��Ҳ��һ��ճ�ϼ����ڿ������ȶ������ڸ���������ȱ����ʱ��������˫���Ͽ������ۺ϶��̻�����ҵ���ñ�ϩ���ij��������һ�������·�Ӧ���Ƶ�����ճ�ϼ�����һ��ȡ���̵Ļ�ѧ����ʽΪ
��Ҳ��һ��ճ�ϼ����ڿ������ȶ������ڸ���������ȱ����ʱ��������˫���Ͽ������ۺ϶��̻�����ҵ���ñ�ϩ���ij��������һ�������·�Ӧ���Ƶ�����ճ�ϼ�����һ��ȡ���̵Ļ�ѧ����ʽΪ2 +HOCH2CH2OH
+HOCH2CH2OH
 +2H2O
+2H2O
 +HOCH2CH2OH
+HOCH2CH2OH| Ũ���� |
| �� |
 +2H2O
+2H2O2 +HOCH2CH2OH
+HOCH2CH2OH
 +2H2O
+2H2O
�� +HOCH2CH2OH
+HOCH2CH2OH| Ũ���� |
| �� |
 +2H2O
+2H2O��3�����齺�dz��õ�ճ�ϼ�������Ҫ�ɷ�Ϊ������ϩ����CH3COOCH=CH2�������ж���ͬ���칹�壬��
 ����֪����
����֪���� �ṹ�����ʲ����ȶ����ڣ���������д���ֺ�-CH=CH-�ṹ����״ͬ���칹��Ľṹ��ʽ��
�ṹ�����ʲ����ȶ����ڣ���������д���ֺ�-CH=CH-�ṹ����״ͬ���칹��Ľṹ��ʽ��CH3CH=CHCOOH��HOCH2CH=CHCHO��CH2=CHCH��OH��CHO��CH3OCH=CHCHO
CH3CH=CHCOOH��HOCH2CH=CHCHO��CH2=CHCH��OH��CHO��CH3OCH=CHCHO
����4����֪��ȩ��Ӧ2CH3OH+HCHO
| һ������ |
 ��������ͨ��ˮ�������ǻ���ȫ��������ȩ������ˮ���õ�����ԭ�ӻ���ǿ��ճ�ϼ�����ϩ����ȩ����д����ȡ����ϩ����ȩ�Ļ�ѧ����ʽ��
��������ͨ��ˮ�������ǻ���ȫ��������ȩ������ˮ���õ�����ԭ�ӻ���ǿ��ճ�ϼ�����ϩ����ȩ����д����ȡ����ϩ����ȩ�Ļ�ѧ����ʽ��

��������1������ϩ�ļӾ۷�Ӧ��д��̼̼˫���Ͽ���Ϊ���ڣ�
��2�����������Ľṹ��֪����������2���ӱ�ϩ�����Ҷ����γɵ�����
��3�����ݴ�����ϩ��д����������������ͬ���칹�壻
��4������Ϣ��֪������ȩ�����ӳɷ�Ӧ��ȩ��̼��˫������1�����Ͽ������Ͽ��������Hԭ�ӽ���ȩ��Oԭ���ϣ�-OR������ȩ�IJ�����̼�ϣ������ﺬ���ǻ���������һ���ӵĴ��������ǻ�֮�����ˮ�������ղ��
��2�����������Ľṹ��֪����������2���ӱ�ϩ�����Ҷ����γɵ�����
��3�����ݴ�����ϩ��д����������������ͬ���칹�壻
��4������Ϣ��֪������ȩ�����ӳɷ�Ӧ��ȩ��̼��˫������1�����Ͽ������Ͽ��������Hԭ�ӽ���ȩ��Oԭ���ϣ�-OR������ȩ�IJ�����̼�ϣ������ﺬ���ǻ���������һ���ӵĴ��������ǻ�֮�����ˮ�������ղ��
����⣺��1��̼̼˫���Ͽ���Ϊ���ڣ�502��������ճ�����õķ���ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��2���������Ľṹ��֪����������2���ӱ�ϩ�����Ҷ����γɵ�������Ӧ����ʽΪ
2 +HOCH2CH2OH
+HOCH2CH2OH
 +2H2O��
+2H2O��
�ʴ�Ϊ��2 +HOCH2CH2OH
+HOCH2CH2OH
 +2H2O��
+2H2O��
��3������ʽ��ͬ���������ụΪͬ���칹�壬
���������ͬ���칹����CH3CH=CHCOOH��CH2=CHCH2COOH��CH2=C��CH3��COOH�ȣ�
�����ǻ���ȩ����ͬ���칹����HOCH2CH=CHCHO��CH2=CHCH��OH��CHO �ȣ�
����ȩ����-OR��RΪ���������ŵ�ͬ���칹����CH3OCH=CHCHO��CH2=C��OCH3��CHO��CH2=CHOCH2CHO�ȣ�
���Խṹʽ�к���-CH=CH-�ṹ����״ͬ���칹����CH3CH=CHCOOH��HOCH2CH=CHCHO��CH2=CHCH��OH��CHO��CH3OCH=CHCHO�ȣ�
�ʴ�Ϊ��CH3CH=CHCOOH��HOCH2CH=CHCHO��CH2=CHCH��OH��CHO��CH3OCH=CHCHO��
��4��������Ϣ��֪������ϩ�ǻ�ȫ��������ȩ������ˮ���õ�����ԭ�ӻ���ǿ��ճ�ϼ�����ϩ����ȩ����Ӧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
 ��
���ʴ�Ϊ��
 ��
����2���������Ľṹ��֪����������2���ӱ�ϩ�����Ҷ����γɵ�������Ӧ����ʽΪ
2
 +HOCH2CH2OH
+HOCH2CH2OH| Ũ���� |
| �� |
 +2H2O��
+2H2O���ʴ�Ϊ��2
 +HOCH2CH2OH
+HOCH2CH2OH| Ũ���� |
| �� |
 +2H2O��
+2H2O����3������ʽ��ͬ���������ụΪͬ���칹�壬
���������ͬ���칹����CH3CH=CHCOOH��CH2=CHCH2COOH��CH2=C��CH3��COOH�ȣ�
�����ǻ���ȩ����ͬ���칹����HOCH2CH=CHCHO��CH2=CHCH��OH��CHO �ȣ�
����ȩ����-OR��RΪ���������ŵ�ͬ���칹����CH3OCH=CHCHO��CH2=C��OCH3��CHO��CH2=CHOCH2CHO�ȣ�
���Խṹʽ�к���-CH=CH-�ṹ����״ͬ���칹����CH3CH=CHCOOH��HOCH2CH=CHCHO��CH2=CHCH��OH��CHO��CH3OCH=CHCHO�ȣ�
�ʴ�Ϊ��CH3CH=CHCOOH��HOCH2CH=CHCHO��CH2=CHCH��OH��CHO��CH3OCH=CHCHO��
��4��������Ϣ��֪������ϩ�ǻ�ȫ��������ȩ������ˮ���õ�����ԭ�ӻ���ǿ��ճ�ϼ�����ϩ����ȩ����Ӧ����ʽΪ
 ��
���ʴ�Ϊ��
 ��
�����������⿼���л���Ľṹ����������ʡ���������ͬ���칹�����д����ϢǨ�Ƶȣ��Ѷ��еȣ�ּ�ڿ���ѧ����֪ʶ��������Ǩ�����á�˼ά������������

��ϰ��ϵ�д�
 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
�����Ŀ
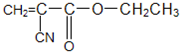 �л��ϳ�ճ�ϼ���������������һ����Ҫ�IJ��ϣ�ճ�ϵĹ���һ���dz�Һ̬��ճ�ϼ�С���ӣ�����ѧ��Ӧת��Ϊ����ӻ�߷��Ӷ��̻�����502������һ�ֿ�ɽ�����Ϊ��-�����ϩ������������¶�ڿ�����ʱ������ˮ������ã�ʹ�䷢��̼̼���ļӾ۷�Ӧ��Ѹ�ٹ̻��������Ӽ��ɰ�ճ������ճ��һ����д����502��������ճ�����õĻ�ѧ����ʽ��
�л��ϳ�ճ�ϼ���������������һ����Ҫ�IJ��ϣ�ճ�ϵĹ���һ���dz�Һ̬��ճ�ϼ�С���ӣ�����ѧ��Ӧת��Ϊ����ӻ�߷��Ӷ��̻�����502������һ�ֿ�ɽ�����Ϊ��-�����ϩ������������¶�ڿ�����ʱ������ˮ������ã�ʹ�䷢��̼̼���ļӾ۷�Ӧ��Ѹ�ٹ̻��������Ӽ��ɰ�ճ������ճ��һ����д����502��������ճ�����õĻ�ѧ����ʽ��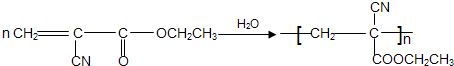
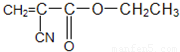
 �����ڿ�������ˮ���·����Ӿ۷�Ӧ��Ѹ�ٹ̻�������ճ�Σ���д����502��������ճ�����õĻ�ѧ����ʽ��
�����ڿ�������ˮ���·����Ӿ۷�Ӧ��Ѹ�ٹ̻�������ճ�Σ���д����502��������ճ�����õĻ�ѧ����ʽ��  ��Ҳ��һ��ճ�ϼ����ڿ������ȶ������ڸ���������ȱ����ʱ��������˫���Ͽ������ۺ϶��̻�����ҵ���ñ�ϩ���ij��������һ�������·�Ӧ���Ƶ�����ճ�ϼ�����һ��ȡ���̵Ļ�ѧ����ʽΪ ��
��Ҳ��һ��ճ�ϼ����ڿ������ȶ������ڸ���������ȱ����ʱ��������˫���Ͽ������ۺ϶��̻�����ҵ���ñ�ϩ���ij��������һ�������·�Ӧ���Ƶ�����ճ�ϼ�����һ��ȡ���̵Ļ�ѧ����ʽΪ �� ����֪����
����֪���� �ṹ�����ʲ����ȶ����ڣ���������д���ֺ�-CH=CH-�ṹ����״ͬ���칹��Ľṹ��ʽ�� ��
�ṹ�����ʲ����ȶ����ڣ���������д���ֺ�-CH=CH-�ṹ����״ͬ���칹��Ľṹ��ʽ�� �� CH3OCH2OCH3+H2O���Է���������ϩ
CH3OCH2OCH3+H2O���Է���������ϩ ��������ͨ��ˮ�������ǻ���ȫ��������ȩ������ˮ���õ�����ԭ�ӻ���ǿ��ճ�ϼ�����ϩ����ȩ����д����ȡ����ϩ����ȩ�Ļ�ѧ����ʽ�� ��
��������ͨ��ˮ�������ǻ���ȫ��������ȩ������ˮ���õ�����ԭ�ӻ���ǿ��ճ�ϼ�����ϩ����ȩ����д����ȡ����ϩ����ȩ�Ļ�ѧ����ʽ�� ��