��Ŀ����
�ش��������⣨����ţ���
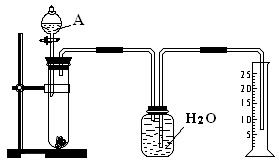
��1�����������У���©����������ƿ����������ƿ������ƽ���ݷ�Һ©��������Ͳ����ȼ�ճף����������ʷ������______�����и������ʷе㲻ͬ���������ʵ�������______�����������д��
��2��������NaOH��������500mL0.2mol/L��NaOH��Һ������������գ�
A�����Ƹ���ҺӦѡ��______mL����ƿ��
B����������ƽ��ȡ______g����NaOH��
C�����ƺõ�NaOH�������500mL�Ĵ��ձ��У�����Լ250mL����ˮ����______��������ȫ�ܽ⣮����ȴ�����º��ձ��е���Һ�ò���������ת��������ƿ��
D������������ˮϴ���ձ�2-3�Σ�����ÿ��ϴ�ӵ���Һ��ע������ƿ������ζ�����ƿ��ʹ��Һ��;��ȣ�
E��������ƿ�м�������ˮ��ֱ��Һ����̶���Լ1-2����ʱ������______�μ�����ˮ��Һ����̶������У��Ǻ�ƿ����ҡ�ȣ������ˮʱҺ�泬���̶��ߣ���ʹ��õ���ҺŨ��______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
F�����ƺõ���Һ______����ܡ����ܡ������ڴ��������ƿ�У�
��1�����������У���©����������ƿ����������ƿ������ƽ���ݷ�Һ©��������Ͳ����ȼ�ճף����������ʷ������______�����и������ʷе㲻ͬ���������ʵ�������______�����������д��
��2��������NaOH��������500mL0.2mol/L��NaOH��Һ������������գ�
A�����Ƹ���ҺӦѡ��______mL����ƿ��
B����������ƽ��ȡ______g����NaOH��
C�����ƺõ�NaOH�������500mL�Ĵ��ձ��У�����Լ250mL����ˮ����______��������ȫ�ܽ⣮����ȴ�����º��ձ��е���Һ�ò���������ת��������ƿ��
D������������ˮϴ���ձ�2-3�Σ�����ÿ��ϴ�ӵ���Һ��ע������ƿ������ζ�����ƿ��ʹ��Һ��;��ȣ�
E��������ƿ�м�������ˮ��ֱ��Һ����̶���Լ1-2����ʱ������______�μ�����ˮ��Һ����̶������У��Ǻ�ƿ����ҡ�ȣ������ˮʱҺ�泬���̶��ߣ���ʹ��õ���ҺŨ��______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
F�����ƺõ���Һ______����ܡ����ܡ������ڴ��������ƿ�У�
��1��©�����ڹ�Һ���롢������ƿ����е��������ܵ�Һ�塢��Һ©�����뻥����Ҫ��Һ�壻����ƿ������Һ������������ڹ̶������ȡ����ƽ�������壬��Ͳ��ȡҺ�壬
�ʴ�Ϊ���٢ۢݣ��ۣ�
��2��A������500mL0.2mol/L��NaOH��Һ������ѡ��500mL����ƿ���ʴ�Ϊ��500��
B������500mL0.2mol/L��NaOH��Һ��Ҫ�������Ƶ�����0.5L��0.2mol/L��40g/mol=4.0g���ʴ�Ϊ��4.0��
C�������ܽ���Ҫ�ò��������裬�����ܽ⣬�ʴ�Ϊ����������
E��������ƿ�м�������ˮ��ֱ��Һ����̶���Լ1-2����ʱ�����ý�ͷ�ιܵμ�����ˮ��Һ����̶������У�
��ˮʱҺ�泬���̶��ߣ�������Һ������ͣ���ʹ��õ���ҺŨ��ƫ�ͣ��ʴ�Ϊ����ͷ�ιܣ�ƫ�ͣ�
F��һЩǿ����Һ�ḯʴ���������ʱ����˻�Ӱ������ƿ�����������ƺõ���Һ���ܳ��ڴ��������ƿ�У��ʴ�Ϊ�����ܣ�
�ʴ�Ϊ���٢ۢݣ��ۣ�
��2��A������500mL0.2mol/L��NaOH��Һ������ѡ��500mL����ƿ���ʴ�Ϊ��500��
B������500mL0.2mol/L��NaOH��Һ��Ҫ�������Ƶ�����0.5L��0.2mol/L��40g/mol=4.0g���ʴ�Ϊ��4.0��
C�������ܽ���Ҫ�ò��������裬�����ܽ⣬�ʴ�Ϊ����������
E��������ƿ�м�������ˮ��ֱ��Һ����̶���Լ1-2����ʱ�����ý�ͷ�ιܵμ�����ˮ��Һ����̶������У�
��ˮʱҺ�泬���̶��ߣ�������Һ������ͣ���ʹ��õ���ҺŨ��ƫ�ͣ��ʴ�Ϊ����ͷ�ιܣ�ƫ�ͣ�
F��һЩǿ����Һ�ḯʴ���������ʱ����˻�Ӱ������ƿ�����������ƺõ���Һ���ܳ��ڴ��������ƿ�У��ʴ�Ϊ�����ܣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ