��Ŀ����
ij̼��غ�̼�������ɵĻ������Ʒ61.4g����ˮ�ܽ��ȡ������Һ��ʮ��֮һ�������л�������һ��Ũ�ȵ�ϡ���ᣬ��ͬʱ��¼�ų�CO2������������ϡ�����������õ���ͼ��ʾ�����ߣ�����˵������ȷ���ǣ�������


| A���ù�����������Ӧ�Ļ�ѧ����ʽ��K2CO3+HCl=KCl+KHCO3��KHCO3+HCl=KCl+H2O+CO2�� | B���������̼Ԫ�ص���������Ϊ8.5% | C���������Ʒ�к�̼���41.4g | D������61.4gԭ�������Ʒ��ּ��ȣ��ų�CO2������Ϊ4.4g |
������A����ͼ��֪����ʼû���������ɣ�������Ӧ��K2CO3+HCl=KCl+KHCO3������12mL����ʱ����ʼ���������ɣ���ʱ������Ӧ��KHCO3+HCl=KCl+H2O+CO2����
B��������32mL����ʱ�����ɵ���������ֵ������̼Ԫ���غ������Ʒ��̼Ԫ�ص���������������̼Ԫ������������
C����Һ��̼���ת��Ϊ̼����أ���������Ϊ12mL��KHCO3��HCl��Ӧ������������Ϊ32mL-12mL=20mL����Ӧ����1��1���У�����Ʒ��̼�����̼����ص����ʵ���֮��=12����20-12��=3��2������̼Ԫ���غ����̼��ص����ʵ���������������������
D������̼����ص����ʵ��������ݷ���ʽ2KHCO3
K2CO3+H2O+CO2�����������̼�����ʵ���������������������
B��������32mL����ʱ�����ɵ���������ֵ������̼Ԫ���غ������Ʒ��̼Ԫ�ص���������������̼Ԫ������������
C����Һ��̼���ת��Ϊ̼����أ���������Ϊ12mL��KHCO3��HCl��Ӧ������������Ϊ32mL-12mL=20mL����Ӧ����1��1���У�����Ʒ��̼�����̼����ص����ʵ���֮��=12����20-12��=3��2������̼Ԫ���غ����̼��ص����ʵ���������������������
D������̼����ص����ʵ��������ݷ���ʽ2KHCO3
| ||
����⣺A����ͼ��֪����ʼû���������ɣ�������Ӧ��K2CO3+HCl=KCl+KHCO3������12mL����ʱ����ʼ���������ɣ���ʱ������Ӧ��KHCO3+HCl=KCl+H2O+CO2������A��ȷ��
B��������32mL����ʱ�����ɵ���������ֵ������̼Ԫ���غ㣬��֪��Ʒ��̼Ԫ�ص�����=2.2g��
��10=6g������Ʒ��̼Ԫ����������=
��100%=9.77%����B����
C����Һ��̼���ת��Ϊ̼����أ���������Ϊ12mL��KHCO3��HCl��Ӧ������������Ϊ32mL-12mL=20mL����Ӧ����1��1���У�����Ʒ��̼�����̼����ص����ʵ���֮��=12����20-12��=3��2������̼Ԫ���غ��֪��̼��ص����ʵ���=
��
=0.3mol������Ʒ��̼��ص�����=0.3mol��138g/mol=41.4g����C��ȷ��
D��̼����ص����ʵ���=
��
=0.2mol�����ݷ���ʽ2KHCO3
K2CO3+H2O+CO2������֪���ɶ�����̼�����ʵ���Ϊ0.2mol��
=0.1mol��������̼������=0.1mol��44g/mol=4.4g����D��ȷ��
��ѡB��
B��������32mL����ʱ�����ɵ���������ֵ������̼Ԫ���غ㣬��֪��Ʒ��̼Ԫ�ص�����=2.2g��
| 12 |
| 44 |
| 6g |
| 61.4g |
C����Һ��̼���ת��Ϊ̼����أ���������Ϊ12mL��KHCO3��HCl��Ӧ������������Ϊ32mL-12mL=20mL����Ӧ����1��1���У�����Ʒ��̼�����̼����ص����ʵ���֮��=12����20-12��=3��2������̼Ԫ���غ��֪��̼��ص����ʵ���=
| 3 |
| 5 |
| 6g |
| 12g/mol |
D��̼����ص����ʵ���=
| 2 |
| 5 |
| 6g |
| 12g/mol |
| ||
| 1 |
| 2 |
��ѡB��
�����������Ի�ѧͼ����ʽ����������㣬����������������ȷ��̼�����̼����ص����ʵ�����ϵ�ǹؼ���ע�������غ�˼����н���Ѷ��еȣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
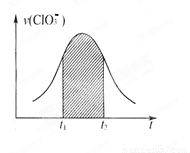
 ��2010?¬������ģ������غ�����������ܷ���������ԭ��Ӧ��ClO3-+HSO3--SO42-+Cl-+H+��δ��ƽ������֪�÷�Ӧ��������c��H+����������ӿ죮��ͼΪ��ClO3-�ڵ�λʱ�������ʵ���Ũ�ȱ仯��ʾ�ĸ÷�Ӧv-tͼ������˵���в���ȷ���ǣ�������
��2010?¬������ģ������غ�����������ܷ���������ԭ��Ӧ��ClO3-+HSO3--SO42-+Cl-+H+��δ��ƽ������֪�÷�Ӧ��������c��H+����������ӿ죮��ͼΪ��ClO3-�ڵ�λʱ�������ʵ���Ũ�ȱ仯��ʾ�ĸ÷�Ӧv-tͼ������˵���в���ȷ���ǣ�������