��Ŀ����
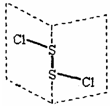
�Ȼ�����(S2Cl2)�ǹ㷺������ҵ����������ӽṹ����ͼ��ʾ��������S2Cl2��һ�ֳȻ�ɫ��Һ�壬��ˮ��ˮ�⣬��������ʹƷ����ɫ�����塣
����˵���������( )
| A��S2Cl2�ĽṹʽΪCl��S��S��Cl |
| B��S2Cl2Ϊ���м��Լ��ͷǼ��Լ��ķǼ��Է��� |
| C��S2Br2��S2Cl2�ṹ���ƣ����Ӽ���������S2Br2>S2Cl2 |
| D��S2Cl2��H2O��Ӧ�Ļ�ѧ����ʽ����Ϊ��2S2Cl2+2H2O=SO2��+3S��+4HCl |
B
�����������������ͼ����ʾ������ȷ��S2Cl2�ĽṹʽΪCl��S��S��Cl��A�ԡ������ڷ��Ӳ���ȫ�Գƣ��������ڼ��Է��ӣ�B�������Ӽ���������ʽ���йأ�ʽ��Խ���Ӽ�������Խ��C�ԡ�����������ԭ���ۣ�D��ȷ��
���㣺���ӵ����ʼ����Ӽ�������
���������ӵļ�������ӵĹ����йأ���ȫ�ԳƵķ���Ϊ�Ǽ��Է��ӣ�����ȫ�ԳƵķ���Ϊ���Է��ӡ�

��ϰ��ϵ�д�
�����Ŀ