��Ŀ����
ijѧ��������l00mL 2mol/L��NaCI��Һ��Ȼ�����ҺŨ������ȷ�ⶨ���Ҳⶨ������һ�в�������ȷ����������Һ�����ʵ���Ũ�ȵ���2mol/L����ô�����ƹ����У����в������ܵ�����ҺŨ��ƫ�͵���
��������ƽ����������NaCI���������ŷ���
�ڽ�NaCl���ձ���ϡ�ͣ�ת�Ƶ��ݻ�ΪlOOmL������ƿ�к�û��ϴ���ձ�
���ڶ���ʱ�۲�Һ�温��
�������ʱ����ˮ�����˿̶ȣ������ý�ͷ�ι���ȥ�����ˮ��ʹ��Һ��Һ��պ���̶�������
��������ƽ����������NaCI���������ŷ���
�ڽ�NaCl���ձ���ϡ�ͣ�ת�Ƶ��ݻ�ΪlOOmL������ƿ�к�û��ϴ���ձ�
���ڶ���ʱ�۲�Һ�温��
�������ʱ����ˮ�����˿̶ȣ������ý�ͷ�ι���ȥ�����ˮ��ʹ��Һ��Һ��պ���̶�������
| A���ڢۢ� | B���ۢ� | C���٢ڢ� | D���٢ڢۢ� |
C
�������������cB��nB/V�ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ�����B����Һ�����V����ġ�������ʱ���ؼ�Ҫ�����ƹ�����������V�����ı仯��������һ�����ʵ���Ũ����Һʱ����nB������ֵС����V������ֵ��ʱ������ʹ������ҺŨ��ƫС����nB������ֵ��V������ֵСʱ������ʹ������ҺŨ��ƫ����Ҫ�Ȼ��Ƶ�������0.1L��2mol/L��58.5g/mol��11.7g�������������ƽ����������NaCI���������ŷ��ˣ���ôʵ�ʳ������Ȼ��ƹ�������С��11.7g��������Ũ��ƫ�ͣ��ڽ�NaCl���ձ���ϡ�ͣ�ת�Ƶ��ݻ�ΪlOOmL������ƿ�к�û��ϴ���ձ��������ʼ��٣�����Ũ��ƫ�ͣ����ڶ���ʱ�۲�Һ�温�ӣ�������ƿ����Һ�����ƫ�٣�������Ũ��ƫ�ߣ��������ʱ����ˮ�����˿̶ȣ������ý�ͷ�ι���ȥ�����ˮ��ʹ��Һ��Һ��պ���̶������У������ʼ��٣�����Ũ��ƫ�ͣ����Դ�ѡC��

��ϰ��ϵ�д�
 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�
�����Ŀ

 Na2S2O3��
Na2S2O3��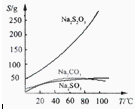
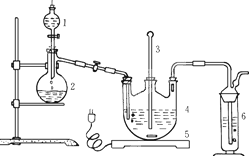
 6I-+Cr2O72-+14H+=3I2+2Cr3++7H2O���ټ��뼸�ε�����Һ������������Na2S2O3��Һ�ζ���������Ӧ�� I2+2S2O32-=2I-+S4O62-���ζ��յ������Ϊ ��
6I-+Cr2O72-+14H+=3I2+2Cr3++7H2O���ټ��뼸�ε�����Һ������������Na2S2O3��Һ�ζ���������Ӧ�� I2+2S2O32-=2I-+S4O62-���ζ��յ������Ϊ ��