��Ŀ����
(��16��) ���������ʵ���Ũ��Ϊa mol/L�ı�����ȥ�ⶨV mL NaOH��Һ�����ʵ���Ũ�ȣ�����д���пհף�
�� ��ʽ�ζ���������ˮϴ����Ӧ�ý��еIJ�����___��___��
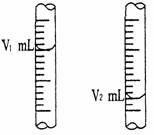 �� ��ͼ����ʽ�ζ�����Һ���ڵζ�ǰ��Ķ�����
�� ��ͼ����ʽ�ζ�����Һ���ڵζ�ǰ��Ķ�����
�����йط��ű�ʾ�ô���NaOH��Һ�����ʵ���Ũ�ȣ�c (NaOH) = ___��___ mol/L��
�� ���ڵζ�ǰ�ζ��ܼ��첿���������ݣ��ζ���ζ��ܼ��첿��������ʧ����ⶨ��NaOH���ʵ���Ũ�Ȼ�ƫ___��___��
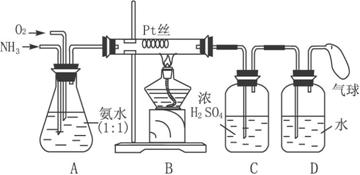
����֪�����к���N2��O2��CO2��H2S�����塣���ж����еζ��������յ㡢�����������������ԭ�������й����ӷ���ʽ��ʾ��
�� �Է�̪Ϊָʾ������Һ�ζ���Һ���� �� Ϊ�յ㡣30s������ɫ��ԭ�� �� ��
�� �Ե���Ϊָʾ������Na2S2O3�ζ�I2(2S2O32��+I2 �� S4O62��+2I��)�� �� Ϊ�յ㣬Լ5min����Һ����ɫ��ԭ�� �� ��
����16�֣�
�ţ�6�֣�������֪���ʵ���Ũ�ȵı�������ϴ2��3�Σ�2�֣�
��c (NaOH) = (V2-V1)a/V ��2�֣� �۸ߣ�2�֣�
�ƣ�10�֣� ������dz��ɫ���ڰ�����ڲ���ɫ����2�֣� �����д���CO2 ��H2S �� CO2+OH��==HCO3���� H2S+OH��==HS��+H2O��4�֣������������ɣ���ͬ��
����ɫǡ����ȥ����2�֣� �����д���O2��O2+4I��+4H+===2H2O+I2��2�֣�

 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д���ÿ��2�֣���16�֣�������Ԫ��X��Y��Z��W��ԭ���������������³�ѹ�£�ֻ��W�ĵ���Ϊ���塣���ǵ�����������Ӧ��ˮ��������Ϊ�ס��ҡ����������ס��ҡ�������ѧ��ѧ�еij������ʣ�����ֻ����������ˮ�����ܺͼס�����Ӧ�õ�������Һ������������Ϣ��д���пհף�
�Ż���W��ԭ�ӽṹʾ��ͼ_________________________________________��
�ƽ��Һͼס����ֱ�Ӧ��õ�����Һ��ϣ��۲쵽��������___________________
___________________ ������Һ���ʱ��������Ӧ�����ӷ���ʽΪ____________________________________________________��
��������ʵ��֤��Z��W�ǽ�����ǿ�����ǣ�ѡ����ţ�__________________________��
| A�����ʵ��۵㣺Z��W2 |
| B�����ԣ������� |
| C������Һ�У�W2��H2Z��2HW��Z |
| D���ȶ��ԣ�HW��H2Z |
F.�ܽ��ԣ�������
����Y���ʺ���������õĽ������缫���õ������Ӳ������Һ�й���ԭ��أ���ԭ��ظ����ĵ缫��ӦʽΪ_______________________________________________ ��
�ɹ�ҵ����XWΪԭ�Ͽ��Խ��������������W2��������Ҫ��Ʒ��д����ҵ����XWΪԭ��������W2�Ļ�ѧ����ʽ_______________________________________________
_______________________________________________����Ҫ����80.0 kg�����ʣ�������ҪXW______________kg��ͬʱ�ɵ�W2_____________m3���������
����16�֣���1������ͭ������ܽ������ܽ���̽�����ʵ���ҳ�����ˮ�����Լӿ��ܽ����ʣ���������������ǣ����Ҫ˵��ԭ��______________________����β�������ˮ���Ƴ�����Ľ�Ũ��CuSO4��Һ____________________��
��2��ϡNa2S��Һ��һ�ֳ�������ζ������AlCl3��Һ��������ζ�Ӿ磬�����ӷ���ʽ��ʾ��ζ�Ӿ�����������Ļ�ѧ��Ӧ______________________________________________
��ij�ռ���Ʒ�к����������������õĿ��������ʣ�Ϊ�˲ⶨ�䴿�ȣ��������µζ�������
| A������Һת����250 mL����ƿ�У���ˮ���̶��ߣ� |
| B������Һ��(���ʽ�ζ���)��ȡ25.00 mL�ռ���Һ����ƿ�в��Ӽ��μ�����ָʾ���� |
| C������ƽ��ȷ��ȡ�ռ���Ʒw g�����ձ��м�����ˮ�ܽ⣻ |
| D�������ʵ���Ũ��Ϊm mol?L��1�ı�H2SO4��Һװ����ʽ�ζ��ܣ�����Һ�棬���¿�ʼ�̶���ΪV1 mL�� |
�ش��������⣺
(1)��ȷ�IJ��������˳����(��д��ĸ)
________��________��________��________��________��
(2)�յ㵽���������________________________��
(3)����ʽ�ζ���û���ñ�H2SO4��ϴ���Բⶨ���Ӱ��________���ζ�ǰ���Ӷ����ζ����Ӷ����Բⶨ���Ӱ��________ ��(�ƫ�ߡ�����ƫ�͡�����Ӱ�족��������������ȷ)��
(4)���ռ�İٷֺ�����________��