��Ŀ����
�л�������A��C5H8O2��������ˮ�������Է�������ͼ��ʾ�ı仯��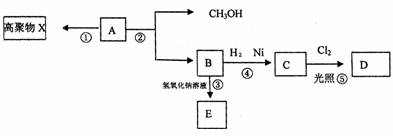
��֪��C��һ�ȴ���Dֻ������
��ش�
��1��A�����к��еĹ����ŵ�����_________��
��2���٢ڢܢݷ�Ӧ������ȡ����Ӧ����___________������ţ���
��3��C�Ľṹ��ʽ __________��X�ṹ��ʽ _____________��
��4��д���ڵķ�Ӧ����ʽ ____________________________________��
��5��C��ͬ�������������������_______�֣�д�����еĽṹ��ʽ_______,__________.
��6����������D�к�����Ԫ�صķ���___________________ ��
��7��17.2gB��������̼��������Һ��Ӧ����״�������ɶ�����̼�����Ϊ________L��
��1��̼̼˫�������� ��2���ڢ�
��3��

��4��
��5��4��CH3CH2COOCH3 CH3COOCH2CH3 HCOOCH2CH2CH3 ����ѡ�����е�������
����ѡ�����е�������
��6��ȡ������D�����м�������������Һ��У��ټ���ϡ���������ԣ��μ���������Һ�����а�ɫ�������ɣ�֤��D�к�����Ԫ�ء� ��7��4.48
��������������л�������A��C5H8O2��������ˮ���ҿ������ɼ״�����˵��AӦ�������࣬��������������ΪA�ܷ����Ӿ۷�Ӧ���ɸ߷��ӻ�����X�����Ը���A�Ļ�ѧʽ��֪��A�����л�����̼̼˫����Aˮ�����ɼ״���B��B������������Һ�����кͷ�Ӧ����E�������������ӳɷ�Ӧ����C��C����������ȡ����Ӧ����D��ӾC��һ�ȴ���Dֻ�����֣���˵��C��������������2����ԭ�ӣ�����C�Ľṹ��ʽ�� ����B�Ľṹ��ʽ��
����B�Ľṹ��ʽ��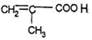 ����A�Ľṹ��ʽ��
����A�Ľṹ��ʽ��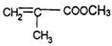 ��
��
���㣺�����л���ṹ��ʽ�������š��л���Ӧ���͡��л�����顢ͬ���칹����ж��Լ�����ʽ����д
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������߿����ۺ���ǿ����ע�ض�ѧ������֪ʶ������ѵ����ͬʱ�����ض�ѧ����������������ⷽ����ָ����ѵ�������������ܽ�ȫ��ؿ���ѧ�����л���ѧ����֪ʶ����˼ά����������˼ά���������ѧ����Ӧ�������ʹ���Ч�ʣ�Ҳ����������ѧ������ѧ������֪ʶ��Ǩ������������Ĺؼ��Ǽ�ס���������ŵĽṹ�������Լ�������֮����ת����Ȼ��������������ü��ɡ�


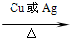 2CH3CHO+2H2O
2CH3CHO+2H2O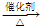
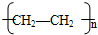 ��
�� CH3COOC2H5+H2O��
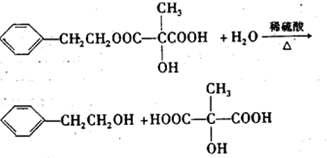
CH3COOC2H5+H2O�� ��2011?��ƽ����ģ��ij�л�������A��������������ã��������ֲ�����������ʹ�������Ľṹ��ʽ��ͼ��ʾ����ش�
��2011?��ƽ����ģ��ij�л�������A��������������ã��������ֲ�����������ʹ�������Ľṹ��ʽ��ͼ��ʾ����ش� �е�һ��
�е�һ��