��Ŀ����
�ϳɰ���ҵ�ĺ��ķ�Ӧ�ǣ�
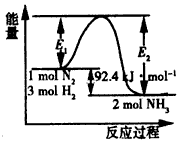
�����仯����ͼ���ش��������⣺
��1���ڷ�Ӧ��ϵ�м����������Ӧ��������E1��E2�ı仯�ǣ�E1�������� ��E2�������������������������С���������䡱��
��2����500�桢2��107Pa�ʹ�����������һ�ܱ������г���0.5mol N2��1.5mol H2����ַ�Ӧ�ų��������������������<������>����=����46.2kJ��
��3�����ڸ÷�Ӧ������˵���У���ȷ����������������������������
A����H>0����S>0������ B����H>0����S< 0���� C����H<0����S>0������ D����H<0����S<0��4����һ������N2��g����H2��g������ 1L�ܱ������У���500�桢2��107Pa�´ﵽƽ�⣬���N2Ϊ0.10mol��H2Ϊ0.30mol��NH3Ϊ0.10mol������������´ﵽƽ��ʱH2ת��ΪNH3��ת���������������������������������¶ȣ�Kֵ�仯���������� �����������С�����䡱����
��5����������4����Ӧ�������ܱ������У�����ߺϳɰ���H2��ת���ʣ����д�ʩ���е������������������� ������ĸ����
A���������а�ԭ�����ٳ���ԭ�������������� B�����������ٳ����������
C���ı䷴Ӧ�Ĵ������������������������� D���������
��1����С�� ��С
��2��<
��3��D
��4��33.3%����С
��5��A��D
����:��

| |||||||||||||||||||||||||||||||||||||||||
 2NH3(g)����H��QKJ��mol��1
2NH3(g)����H��QKJ��mol��1

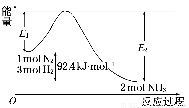
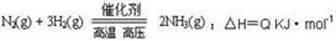
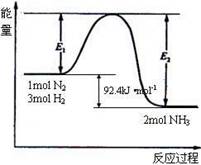
 2NH3(g) ��H=Q kJ/mol����Ӧ�����������仯��ͼ��ʾ���ش��������⣺
2NH3(g) ��H=Q kJ/mol����Ӧ�����������仯��ͼ��ʾ���ش��������⣺