��Ŀ����
0��3molij�л����ڳ���0��6mol O2���ܱ�������ȼ�գ�����Ϊ��CO2��CO��H2O��g����ɵĻ����û���ᆳ��Ũ�����Ũ��������16��2g����ͨ�����ȵ�CuO��ַ�Ӧ��ʹCuOʧ��4��8 g�����ͨ����ʯ�ң���ʯ������26��4 g����ͨ�������ƶ�
��1����������CO2��CO��H2O��g�������ʵ���֮��Ϊ___________________��
��2�����л���ķ���ʽΪ___________________��
��3����0��1mol�ĸ��л�����������������ȫ��Ӧ�����ɱ�״���µ�����2��24L������л���Ľṹ��ʽΪ___________________����0��1mol�ĸ��л�����������������ȫ��Ӧ�����ɱ�״���µ�����
1��12L������л���Ľṹ��ʽΪ___________________��
��1����������CO2��CO��H2O��g�������ʵ���֮��Ϊ___________________��
��2�����л���ķ���ʽΪ___________________��
��3����0��1mol�ĸ��л�����������������ȫ��Ӧ�����ɱ�״���µ�����2��24L������л���Ľṹ��ʽΪ___________________����0��1mol�ĸ��л�����������������ȫ��Ӧ�����ɱ�״���µ�����
1��12L������л���Ľṹ��ʽΪ___________________��
��1��1��1��3
��2��C2H6O2
��3��CH2OHCH2OH��CH3OCH2OH
��2��C2H6O2
��3��CH2OHCH2OH��CH3OCH2OH

��ϰ��ϵ�д�
�����Ŀ
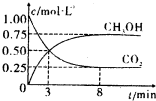 ��2012?��ׯ��ģ��̼��̼�Ļ����������������������е�Ӧ�÷dz��㷺����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӣ�
��2012?��ׯ��ģ��̼��̼�Ļ����������������������е�Ӧ�÷dz��㷺����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӣ�
 CO2��SO2��NOx�ǶԻ���Ӱ��ϴ�����壬���ƺ�����CO2��SO2��NOx�ǽ������ЧӦ����������⻯ѧ��������Ч;����
CO2��SO2��NOx�ǶԻ���Ӱ��ϴ�����壬���ƺ�����CO2��SO2��NOx�ǽ������ЧӦ����������⻯ѧ��������Ч;���� CO+3H2
CO+3H2