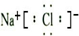��Ŀ����
��ˮ�в������зḻ�ķǽ���Ԫ����Դ����Cl��Br��I �ȣ��������зḻ�Ľ���Ԫ����Դ����Na��Mg��
Fe��Cr�ȣ���
(1)��ˮɹ�ε�ԭ���ǣ�________________��д���Ȼ��Ƶĵ���ʽ��_____________����Na��Clͬ���ڣ��Ҽ����Ӱ뾶��С�����ӽṹʾ��ͼ��____________��
(2)ɹ���Ĵ��γ�����MgSO4��CaSO4�����ʣ�Ϊ�˵õ������Σ����ᴿ���̲�������ͼ����Լ���˳���ǣ����ܽ⣻��_____________���ۼӹ���Na2CO3��Һ����_____________���ݹ��˳�ȥ���ʣ�
��___________���������ᾧ��
(3)ɹ�εõ���ĸҺ����±���к��зḻ��þԪ�أ������г�����Fe2+��Cr3+�ȣ���Ҫ����þʹ��ת��ΪMgCl2���塣�й�����
Fe��Cr�ȣ���
(1)��ˮɹ�ε�ԭ���ǣ�________________��д���Ȼ��Ƶĵ���ʽ��_____________����Na��Clͬ���ڣ��Ҽ����Ӱ뾶��С�����ӽṹʾ��ͼ��____________��
(2)ɹ���Ĵ��γ�����MgSO4��CaSO4�����ʣ�Ϊ�˵õ������Σ����ᴿ���̲�������ͼ����Լ���˳���ǣ����ܽ⣻��_____________���ۼӹ���Na2CO3��Һ����_____________���ݹ��˳�ȥ���ʣ�
��___________���������ᾧ��
(3)ɹ�εõ���ĸҺ����±���к��зḻ��þԪ�أ������г�����Fe2+��Cr3+�ȣ���Ҫ����þʹ��ת��ΪMgCl2���塣�й�����

Ϊ����Ч��ȥ�������ӣ��ֲ������µ��������ӣ��������㡰��ɫ��ѧ�����������Լ�ѡ��Ͳ����ǣ�
���ȼ�____________��Ŀ����____________��
���ټ�____________��Ŀ����____________��
�۹��˺�Ϊ���ܵõ�����MgCl2���壬���õIJ��������ǣ�____________________
���ȼ�____________��Ŀ����____________��
���ټ�____________��Ŀ����____________��
�۹��˺�Ϊ���ܵõ�����MgCl2���壬���õIJ��������ǣ�____________________
(1)�������ᾧ�� ��
��
(2)�ڼӹ���BaCl2��Һ���ӹ���NaOH����ϡHCl����Һ������Һ������
(3)��H2O2����Fe2+������Fe3+����MgO��������ҺpH��5.0<pH<9.5��ʹ����������ȫ������
�ۼ����ᣬ�����Ի����������ᾧ����ֹˮ��
 ��
��
(2)�ڼӹ���BaCl2��Һ���ӹ���NaOH����ϡHCl����Һ������Һ������
(3)��H2O2����Fe2+������Fe3+����MgO��������ҺpH��5.0<pH<9.5��ʹ����������ȫ������
�ۼ����ᣬ�����Ի����������ᾧ����ֹˮ��

��ϰ��ϵ�д�
�����Ŀ
��ˮ�в������зḻ�ķǽ���Ԫ����Դ������Cl��Br��I�ȣ��������зḻ�Ľ���Ԫ����Դ����Na��Mg��Fe��Cr�ȣ���
��1����ˮɹ�ε�ԭ���ǣ�______��д���Ȼ��Ƶĵ���ʽ��______����Na��Clͬ���ڣ��Ҽ����Ӱ뾶��С�����ӽṹʾ��ͼ��
��2��ɹ���Ĵ��γ�����MgSO4��CaSO4�����ʣ�Ϊ�˵õ������Σ����ᴿ���̲�������ͼ����Լ���˳���ǣ����ܽ⣬��______���ۼӹ���Na2CO3��Һ����______���ݹ��˳�ȥ���ʣ���______���������ᾧ��
��3��ɹ�εõ���ĸҺ����±���к��зḻ��þԪ�أ������г�����Fe2+��Cr3+�ȣ�Ϊ����þʹ��ת����MgCl2���壮
�й����ϣ�
Ϊ����Ч��ȥ�������ӣ��ֲ������µ��������ӣ��������㡰��ɫ��ѧ�����������Լ�ѡ��Ͳ����ǣ�
���ȼ�______��Ŀ����______��
���ټ�______��Ŀ����______��
�۹��˺�Ϊ�ܵõ�����MgCl2���壬���õIJ��������ǣ�______��
��1����ˮɹ�ε�ԭ���ǣ�______��д���Ȼ��Ƶĵ���ʽ��______����Na��Clͬ���ڣ��Ҽ����Ӱ뾶��С�����ӽṹʾ��ͼ��
��2��ɹ���Ĵ��γ�����MgSO4��CaSO4�����ʣ�Ϊ�˵õ������Σ����ᴿ���̲�������ͼ����Լ���˳���ǣ����ܽ⣬��______���ۼӹ���Na2CO3��Һ����______���ݹ��˳�ȥ���ʣ���______���������ᾧ��
��3��ɹ�εõ���ĸҺ����±���к��зḻ��þԪ�أ������г�����Fe2+��Cr3+�ȣ�Ϊ����þʹ��ת����MgCl2���壮
�й����ϣ�
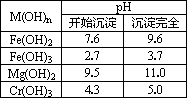
| M��OH��n | pH | |
| ��ʼ���� | ������ȫ | |
| Fe��OH��2 | 7.6 | 9.6 |
| Fe��OH��3 | 2.7 | 3.7 |
| Mg��OH��2 | 9.5 | 11.0 |
| Cr��OH��3 | 4.3 | 5.0 |
���ȼ�______��Ŀ����______��
���ټ�______��Ŀ����______��
�۹��˺�Ϊ�ܵõ�����MgCl2���壬���õIJ��������ǣ�______��