��Ŀ����
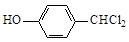
�л���G���Ʊ�Һ�����ϵ��м���֮һ����ṹ��ʽΪ��
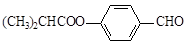 G��һ�ֺϳ�·�����£�
G��һ�ֺϳ�·�����£�
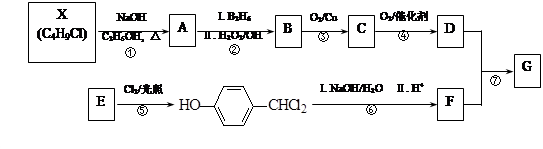
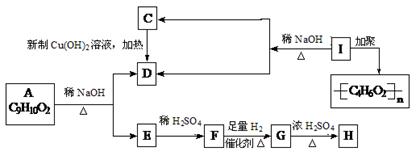
���У�A��F�ֱ����һ���л�������ϳ�·���еIJ��ֲ��P��Ӧ��������ȥ��
��֪��X�ĺ˴Ź�������ֻ��1�ַ壻RCH��CH2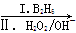 RCH2CH2OH��ͨ����ͬһ��̼ԭ�������������ǻ����ȶ�������ˮ�γ��ʻ�����ش��������⣺
RCH2CH2OH��ͨ����ͬһ��̼ԭ�������������ǻ����ȶ�������ˮ�γ��ʻ�����ش��������⣺
��1��A�Ľṹ��ʽ�� ��C�й����ŵ������� ��
��2��B�������� ���ڢ١��߲�������ȡ����Ӧ���� ������ţ���
��3��д����Ӧ�ݵĻ�ѧ����ʽ�� ��
��4���ڢ߲���Ӧ�Ļ�ѧ����ʽ�� ��
��5��G���������õ�Y��C11H12O4����д��ͬʱ��������������Y������ͬ���칹��Ľṹ��ʽ ��
a.�����ϵ�һ�ȴ�����2�֣�b.ˮ�����ɶ�Ԫ����ʹ���
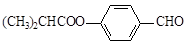 G��һ�ֺϳ�·�����£�
G��һ�ֺϳ�·�����£�
���У�A��F�ֱ����һ���л�������ϳ�·���еIJ��ֲ��P��Ӧ��������ȥ��
��֪��X�ĺ˴Ź�������ֻ��1�ַ壻RCH��CH2
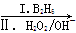 RCH2CH2OH��ͨ����ͬһ��̼ԭ�������������ǻ����ȶ�������ˮ�γ��ʻ�����ش��������⣺
RCH2CH2OH��ͨ����ͬһ��̼ԭ�������������ǻ����ȶ�������ˮ�γ��ʻ�����ش��������⣺��1��A�Ľṹ��ʽ�� ��C�й����ŵ������� ��
��2��B�������� ���ڢ١��߲�������ȡ����Ӧ���� ������ţ���
��3��д����Ӧ�ݵĻ�ѧ����ʽ�� ��
��4���ڢ߲���Ӧ�Ļ�ѧ����ʽ�� ��
��5��G���������õ�Y��C11H12O4����д��ͬʱ��������������Y������ͬ���칹��Ľṹ��ʽ ��
a.�����ϵ�һ�ȴ�����2�֣�b.ˮ�����ɶ�Ԫ����ʹ���
��1��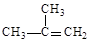 ��ȩ�� ��2��2-��-1-������ �ݢޢ�
��ȩ�� ��2��2-��-1-������ �ݢޢ�
��3��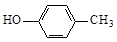 ��2Cl2
��2Cl2 
 �� 2HCl
�� 2HCl
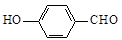
��4�� ��
��
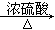
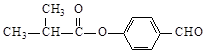 ��H2O
��H2O
��5��

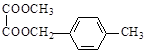
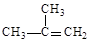 ��ȩ�� ��2��2-��-1-������ �ݢޢ�
��ȩ�� ��2��2-��-1-������ �ݢޢ���3��
 ��2Cl2
��2Cl2 
 �� 2HCl
�� 2HCl��4��
 ��
��
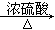
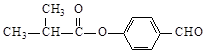 ��H2O
��H2O��5��


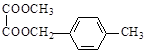
���������X�ķ���ʽΪC4H9Cl��˴Ź�������ֻ��1�ַ壬������ֻ��һ�ֻ�ѧ�������⣬˵��XΪ2-��-2-�ȱ��飻X
 A����ȥ��Ӧ����AΪ(CH3) 2C��CH2��A
A����ȥ��Ӧ����AΪ(CH3) 2C��CH2��A B�Ǽӳɷ�Ӧ����BΪ(CH3) 2CHCH2OH��B
B�Ǽӳɷ�Ӧ����BΪ(CH3) 2CHCH2OH��B C��������Ӧ����CΪ(CH3) 2CHCHO��C
C��������Ӧ����CΪ(CH3) 2CHCHO��C D��������Ӧ����DΪ(CH3) 2CHCOOH��������֪E
D��������Ӧ����DΪ(CH3) 2CHCOOH��������֪E
 ����ȡ����Ӧ����EΪ
����ȡ����Ӧ����EΪ ��
��
 F����ˮ�ⷴӦ����FΪ
F����ˮ�ⷴӦ����FΪ ��������֪D+F
��������֪D+F GΪ������Ӧ����GΪ
GΪ������Ӧ����GΪ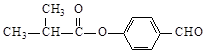 ��
��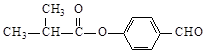 ��ȩ�����������ᣬ��YΪ(CH3)2COO(C6H4)COOH��
��ȩ�����������ᣬ��YΪ(CH3)2COO(C6H4)COOH����1��A�Ľṹ��ʽ
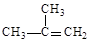 ��CΪ(CH3) 2CHCHO�����������Ϊȩ����2��BΪ(CH3) 2CHCH2OH������Ϊ2-��-1-���������Ϸ���֪�١�����ȡ����ӦΪ�ݢޢߣ�3����������ǹ�������������������ȡ����Ӧ���ʷ�Ӧ����ʽ
��CΪ(CH3) 2CHCHO�����������Ϊȩ����2��BΪ(CH3) 2CHCH2OH������Ϊ2-��-1-���������Ϸ���֪�١�����ȡ����ӦΪ�ݢޢߣ�3����������ǹ�������������������ȡ����Ӧ���ʷ�Ӧ����ʽ ��2Cl2
��2Cl2 
 �� 2HCl��4���������D��F����������Ӧ���ʷ�Ӧ����ʽ
�� 2HCl��4���������D��F����������Ӧ���ʷ�Ӧ����ʽ ��
��
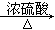
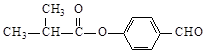 ��H2O��5��YΪ(CH3)2COO(C6H4)COOH��������ab��������ͬ���칹��Ϊ
��H2O��5��YΪ(CH3)2COO(C6H4)COOH��������ab��������ͬ���칹��Ϊ

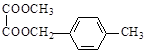

��ϰ��ϵ�д�
 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
�����Ŀ
 B
B C
C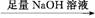 D
D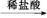 E
E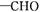 +
+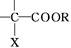
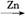
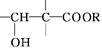 +Zn(OH)X
+Zn(OH)X
 G+Zn(OH)Br
G+Zn(OH)Br A+2M(����,F��G��M�ֱ����һ���л���)
A+2M(����,F��G��M�ֱ����һ���л���)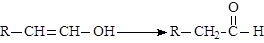
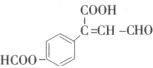
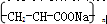
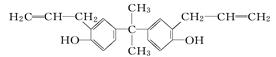
 �ǽ���������ֲ����ʱ����ϸ����ֳ��õ�ҩ���ṹ��ʽ��ͼ��ʾ������˵����ȷ����
�ǽ���������ֲ����ʱ����ϸ����ֳ��õ�ҩ���ṹ��ʽ��ͼ��ʾ������˵����ȷ����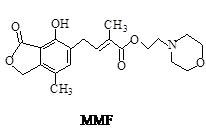
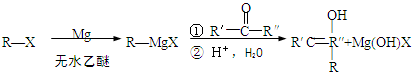
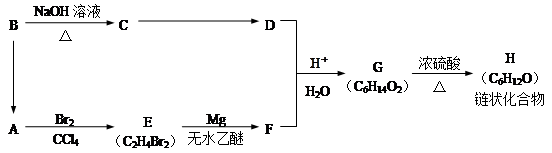
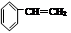 ���ۺ϶��ɣ���һ�ֶ�����ϣ��㷺Ӧ����ʳƷ��װ�������豸�������ճ����������С�д���� D�ͱ�Ϊ��Ҫԭ���Ʊ�����ϩ��
���ۺ϶��ɣ���һ�ֶ�����ϣ��㷺Ӧ����ʳƷ��װ�������豸�������ճ����������С�д���� D�ͱ�Ϊ��Ҫԭ���Ʊ�����ϩ�� B ����
B ����