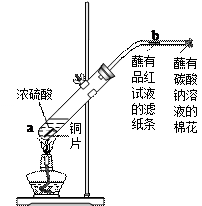
��Ŀ����
�� 6�֣�����ͼ����һ֧�Թ��з���һС��ͭƬ���ټ�������Ũ���ᣬȻ����Թ̶ܹ�������̨�ϡ���һС��պ��Ʒ����Һ����ֽ������е�����Ƥ���IJ������С������Թܿڣ��ڲ������ܿڴ�����һ��պ��Na2CO3��Һ���������Թܼ��ȣ��۲������Թ��е�Һ������ʱ��ֹͣ���ȡ�

�ش��������⣺
��1���Թ��е�Һ�巴Ӧһ��ʱ���b����ֽ���ı仯Ϊ _��
���Թ��з�Ӧֹͣ�������ܷ���պ��Ʒ����Һ����ֽ�����ȣ���ֽ���ı仯Ϊ ��
��2�����Թ��е�Һ����ȴ���Թ��ϲ�Һ�嵹ȥ����������������ˮ���ɹ۲���Һ�� ɫ��
��3���������ܿ�պ��Na2CO3��Һ��������������� ���йط�Ӧ�Ļ�ѧ����ʽΪ ��
����:

 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д���12�֣�
A����ͼ��Ԫ�����ڱ��е�ǰ�����ڣ��١���Ϊ��Ӧ��Ԫ�أ������ѡ����ʵ�Ԫ�ػش����⣺
|
| |||||||||||||||||
| �� | �� | �� | �� | �� | |||||||||||||
| �� | �� | �� | |||||||||||||||
��1������Ԫ��ԭ�ӵ���Χ�����Ų�������Ԫ�����ڱ��ɻ���Ϊ�������Ԫ��λ�����ڱ�
�� ����
��2���ڡ�����Ԫ���γɵĻ�����Ŀռ乹��Ϊ ��������ԭ�ӵ��ӻ�����Ϊ ��
��3��д��Ԫ�آ��̬ԭ�ӵĵ����Ų�ʽ ��
��4���٢���Ԫ���γɵ���Ļ������� ��д�����ţ���Ϊ�ȵ����塣
��5��Ԫ�آ���CO���γɵ�X��CO��5�ͻ�����û����ﳣ���³�Һ̬���۵�Ϊ��20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��жϸû����ᄃ������___ ���壨������ͣ���
��6��Ԫ�آ�����ӵ��������ﲻ����ˮ���������ڰ�ˮ�У���������NH3���ϵ�������
Ϊ ��
��7�� �����ߵľ����ڲ�ͬ�¶��������ֶѻ���ʽ�������� ������ͼ��ʾ��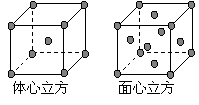 ������������������������������ʵ�ʺ��е�ԭ�Ӹ���֮��Ϊ ��
������������������������������ʵ�ʺ��е�ԭ�Ӹ���֮��Ϊ ��
B����12�֣��Ҵ�����Ҫ�Ļ�����Ʒ��Һ��ȼ�ϣ������������з�Ӧ��ȡ�Ҵ�
2CO2(g)+6H2(g) ![]() CH3CH2OH(g)+3H2O(g)�٣�25��ʱ�� K=2.95��1011
CH3CH2OH(g)+3H2O(g)�٣�25��ʱ�� K=2.95��1011
2CO(g)+4H2(g) ![]() CH3CH2OH(g)+H2O(g)�ڣ�25��ʱ�� K=1.71��1022
CH3CH2OH(g)+H2O(g)�ڣ�25��ʱ�� K=1.71��1022
(1)д����Ӧ�ٵ�ƽ�ⳣ������ʽK=__________________��
(2)������ͬʱ����Ӧ���뷴Ӧ����ȣ�ת���̶ȸ������________����CO2Ϊԭ�Ϻϳ��Ҵ����ŵ��� (д��һ������)��
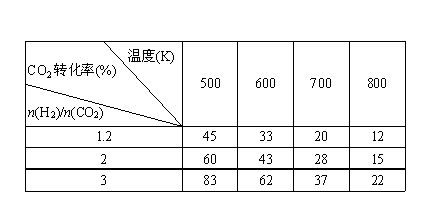 |
(3)��һ��ѹǿ�£���÷�Ӧ�ٵ�ʵ���������±���
���ݱ������ݷ�����
���¶����ߣ�Kֵ______(���������С�����䡱)��
�������̼��[ n��H2��/n��CO2��]���������Ҵ� (�����������������)��
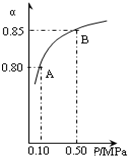
 (4)����ͼ����������ͼ˵��ѹǿ�仯�Է�Ӧ�ٵĻ�ѧƽ���Ӱ�졣��������ͼ�б�������������ʾ����������
(4)����ͼ����������ͼ˵��ѹǿ�仯�Է�Ӧ�ٵĻ�ѧƽ���Ӱ�졣��������ͼ�б�������������ʾ����������
 ��������Ҫ�ɷ�ΪFeS2���ǹ�ҵ��ȡ�������Ҫԭ�ϣ������ղ���ΪSO2��Fe2O3��
��������Ҫ�ɷ�ΪFeS2���ǹ�ҵ��ȡ�������Ҫԭ�ϣ������ղ���ΪSO2��Fe2O3��
 ��
��