��Ŀ����
��A����B����C2����D��E��F��G��H�ֱ��ʾ����18�����ӵİ�����(���ӻ����)����ش�
(1)AԪ����________��BԪ����___________��CԪ����__________(��Ԫ�ط��ű�ʾ)��
(2)D��������Ԫ����ɵ�˫ԭ�ӷ��ӣ���ṹʽ��__________________��
(3)E�����к�18�����ӵ���������������ǿ�ķ��ӣ������ʽ��________________��
(4)F��������Ԫ����ɵ���ԭ�ӷ���______�������ʽ��________________��
(5)G�����к���4��ԭ�ӣ���������ˮ��ԭ����________________
(6)A����C2���γɵĻ�����ˮ��Һ��_____�ԣ���ԭ���ǣ������ӷ���ʽ��ʾ��___________����ˮ��Һ������Ũ���ɴ�С��˳����_____________��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д��ȱ���Ⱦ�ϡ�ҽҩ��ҵ���������챽�ӡ������ȱ��������������ӵ��л��м��塣ʵ�������Ʊ��ȱ���װ������ͼ��ʾ�����мг�����������װ����ȥ��
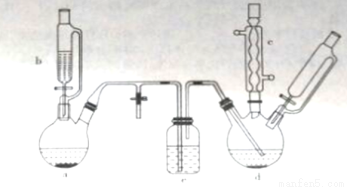
��ش��������⣺
(1)����a��ʢ��KMnO4���壬����b��ʢ��Ũ���ᡣ������b�еĻ�����ʹŨ���Ỻ�����£��ɹ۲쵽����a�ڵ�������__________�������ӷ���ʽ��ʾ�����������ԭ��_______________��
(2)����b��ಣ�����ܵ�������_____________��
(3)����d��ʢ�б���FeCl3��ĩ���壬����a�����ɵ����徭������e���뵽����d�С�
������e��������_________����ʢװ���Լ�������_____________��
������d�еķ�Ӧ���й����У������¶���40~60�棬�Լ��ٸ���Ӧ����������d�ļ��ȷ�ʽ�����___���ȣ����ŵ���____________��
(4)����c��������______________��
(5)�÷����Ʊ����ȱ��к��кܶ����ʣ���ҵ�����У�ͨ��ˮϴ��ȥFeCl3��HCl������Cl2��Ȼ��ͨ����ϴ��ȥCl2����ϴ��ͨ����Һ�õ����ȱ����л�����������ɷּ��е����±���
�л��� | �� | �ȱ� | �ڶ��ȱ� | ����ȱ� | �Զ��ȱ� |
�е�/�� | 80 | 132.2 | 180.4 | 173.0 | 174.1 |
�Ӹ��л�����������ȡ�ȱ�ʱ����������ķ������ռ�_________�����õ���֡�
(6)ʵ�ʹ�ҵ�����У�������ʧ���±���
��ʧ��Ŀ | �����ӷ� | ���ȱ� | ���� | �ϼ� |
����ʧ����kg/t�� | 28.8 | 14.5 | 56.7 | 100 |
ijһ��Ͷ������13t�������Ƶ��ȱ�________t������һλС������
�������������ʵ�Ľ�����ȷ����
ѡ�� | �������ʵ | ���� |
A | ���ȵĴ�����Һϴȥ���� | Na2CO3��ֱ�������۷�Ӧ |
B | Ư���ڿ����о��ñ��� | Ư���е�CaCl2������е�CO2��Ӧ����CaCO3 |
C | ��Mg��OH��2����Һ�еμ�CuSO4��Һ��������ɫ���� | Cu��OH��2���ܶȻ���Mg��OH��2��С��Mg��OH��2ת��ΪCu��OH��2 |
D | ʩ��ʱ����ľ�ң���Ч�ɷ�ΪK2CO3��������NH4Cl���ʹ�� | K2CO3��NH4Cl��Ӧ����ʧ�ط� |
A. A B. B C. C D. D



