��Ŀ����
I.��֪������������ԭ��Ӧ��Zn+2HNO3+NH4NO3��N2��+Zn(NO3)2+3H2O����
��1���÷�Ӧ�Ļ�ԭ��Ϊ������������������ԭ����Ϊ������������������д��ѧʽ��
��2����������N2�ڱ�״�������Ϊ2.24L����Ӧ��ת�Ƶĵ�����Ϊ��������NA��
II.��6�֣���ѧ�����ǵ�����������أ����û�ѧ����ʽ�������������е�ʵ����
��1��ʢ��NaHCO3�ĸɷ�������������
��2�����ʲ˵��ġ���𡱹��գ������ȵIJ˵�����ˮ�м�����ȴ
��3�����������õ�����ˮ���뾭��̫����ɹ�����ʹ��
I.(6��)��1��Zn��NH4NO3�����֣�������N2�����֣����� ����2��0.5��2�֣�
II.��6�֣�
��1��2NaHCO3 Na2CO3��CO2����H2O
Na2CO3��CO2����H2O
(2)3Fe��4H2O  Fe3O4��4H2 (3)2HClO
Fe3O4��4H2 (3)2HClO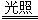 2HCl��O2�� ��ÿ��2�֣�
2HCl��O2�� ��ÿ��2�֣�
��������
���������I. ��1��Zn��NH4NO3��NH4+�е�NԪ�أ����ϼ����ߣ��Ƿ�Ӧ�Ļ�ԭ����N2���������������ǻ�ԭ���
��2��2.24L��0.1mol����Ӧ��ת�Ƶĵ�����Ϊ0.5NA��
II. ��1������NaHCO3�����ֽ�������CO2��ԭ��������ʽΪ2NaHCO3 Na2CO3��CO2����H2O��
Na2CO3��CO2����H2O��
��2������Fe�ڸ�����������ˮ������Ӧ��ԭ��������ʽΪ3Fe��4H2O  Fe3O4��4H2��
Fe3O4��4H2��
��3����������������ˮ�к���HClO����HClO����ǿ�����Ե������ֽ⣬��ɹ�����ʹ�ã�����ʽΪ2HClO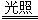 2HCl��O2����
2HCl��O2����
���㣺������ԭ��Ӧ��Ӧ�� ��ѧ������
���������⿼��������ԭ��Ӧ��Ӧ�úͻ�ѧ����������֪ʶ��Ҫ��ѧ�����з����ͽ��������������Ѷ��еȡ�