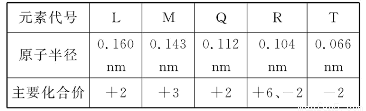题目内容
下表为元素周期表的一部分,参照元素①~⑦在表中的位置,请用化学用语回答下列问题:

(1)④、⑤、⑦的原子半径由大到小的顺序为 (用元素符号表示,下同)。
(2)⑥和⑦的最高价含氧酸的酸性强弱为 > 。
(3)①、②两种元素按原子个数之比为1∶1组成的常见液态化合物,在酸性溶液中能将Fe2+氧化,写出该反应的离子方程式 。
(4)由表中元素形成的物质可发生如图中的反应,其中B、C、G是单质,B为黄绿色气体,D溶液显碱性。

①写出D溶液与G反应的化学方程式 。
②写出检验A溶液中溶质的阴离子的方法: 。
③常温下,若电解1 L 0.1 mol/L的A溶液,一段时间后测得溶液pH为12(忽略溶液体积变化),则该电解过程中转移电子的物质的量为 mol。
④若上图中各步反应均为恰好完全转化,则混合物X中含有的物质有 。
(1)Na>Cl>F (2)HClO4 H2SO4
(3)H2O2+2Fe2++2H+=2Fe3++2H2O
(4)①2Al+2NaOH+2H2O=2NaAlO2+3H2↑
②取少量A溶液滴加几滴(稀硝酸酸化的)硝酸银溶液有白色沉淀生成
③0.01 ④Al(OH)3、H2O、NaCl
【解析】根据元素在周期表中的位置可以确定各元素。
(1)由元素周期表中原子半径递变规律可知,原子半径Na>Cl>F。
(2)非金属性S<Cl,故酸性H2SO4<HClO4。
(3)H、O按1∶1组成H2O2,可氧化Fe2+:
H2O2+2Fe2++2H+=2Fe3++2H2O
(4)黄绿色气体B为Cl2,由图中转化关系可知A为NaCl,C为H2,D为NaOH,由D(NaOH)可以和G反应生成C(H2)可知,G为Al。
①2Al+2NaOH+2H2O=2NaAlO2+3H2↑。
②检验Cl-应取少量试液,滴加硝酸酸化的硝酸银溶液。
③2NaCl+2H2O 2NaOH+H2↑+Cl2↑,产生OH-的物质的量为:0.01 mol/L×
2NaOH+H2↑+Cl2↑,产生OH-的物质的量为:0.01 mol/L×
1 L=0.01 mol,每生成1 mol NaOH转移1 mol电子。故转移电子为0.01 mol。
④由各步反应恰好完全转化可知HCl和NaAlO2溶液等物质的量混合,发生反应:HCl+NaAlO2+H2O=Al(OH)3↓+NaCl。