��Ŀ����
3��${\;}_{92}^{235}U$����Ҫ�Ĺ�ҵԭ�ϣ�����˵������ȷ���ǣ�������| A�� | ${\;}_{92}^{235}U$ԭ�Ӻ��к���92������ | B�� | ${\;}_{92}^{235}U$ԭ�Ӻ�����92������ | ||
| C�� | ${\;}_{92}^{235}U$��${\;}_{92}^{238}U$����Ϊ���� | D�� | ${\;}_{92}^{235}U$��${\;}_{92}^{238}U$��������ͬ |
���� 92235U��U���½ǵ�����Ϊ�����������Ͻǵ�����Ϊ��������ԭ����������=������+��������������ͬ�������IJ�ͬԭ�ӻ�Ϊͬλ�أ�
��� �⣺A��92235U������Ϊ92����A��ȷ��
B��92235U������Ϊ92��������=���������=92����B��ȷ��
C��92235U��92238U��������ͬ����������ͬ���Dz�ͬ�ĺ��أ������ǻ��ƣ���C����
D��92235U��92238U��������ͬ����������ͬ����D��ȷ��
��ѡC��
���� ���⿼��ԭ�ӵĹ��ɼ�ͬλ�أ���ȷԭ���е����ֱ�ʾ�����弰ͬλ�ء����صĸ���ɽ���ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
13�������£����и���������ָ����Һ��һ���ܴ���������ǣ�������
| A�� | c��H+��/c��OH-��=1012����Һ�У�NH${\;}_{4}^{+}$��Al3+��NO${\;}_{3}^{-}$��Cl- | |
| B�� | ��ˮ�����c��H+��=1��10-14mol•L-1����Һ�У�Ca2+��K+��Cl-��F- | |
| C�� | pH=7����Һ�У�K+��CO32-��SO42-��Cl- | |
| D�� | pH=1����Һ�У�Fe2+��NO${\;}_{3}^{-}$��SO${\;}_{4}^{2-}$��Na+ |
14���Դ�п����������ʯī�����������ijŨ�ȵ�����п��Һ��������������ڵ缫�ϣ�ͨ��һ��ʱ��رյ�Դ��Ѹ�ٳ�ȥ�缫������������û����ģ������ڵ������Һ�м���4.95g Zn��OH��2���壬��ǡ����ʹ��Һ�ָ���ԭŨ�ȣ��������������У������������������Ϊ����״������������
| A�� | 0.56L | B�� | 1.12L | C�� | 2.24L | D�� | 3.36L |
15��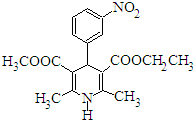 ��Ⱥ��ƽƬ���������Ƹ�Ѫѹ������Ҫ�ɷֵĽṹ��ʽ��ͼ��ʾ�����й������ֻ������˵����ȷ���� ��������
��Ⱥ��ƽƬ���������Ƹ�Ѫѹ������Ҫ�ɷֵĽṹ��ʽ��ͼ��ʾ�����й������ֻ������˵����ȷ���� ��������
 ��Ⱥ��ƽƬ���������Ƹ�Ѫѹ������Ҫ�ɷֵĽṹ��ʽ��ͼ��ʾ�����й������ֻ������˵����ȷ���� ��������
��Ⱥ��ƽƬ���������Ƹ�Ѫѹ������Ҫ�ɷֵĽṹ��ʽ��ͼ��ʾ�����й������ֻ������˵����ȷ���� ��������| A�� | �����ʵķ���ʽΪC18H20N2O6 | |
| B�� | �����ʿ��Ժ�������Һ����������Ӧ | |
| C�� | ���������ڷ����廯���������ˮ | |
| D�� | �����������������²�����ˮ�ⷴӦ |
12������������ͭƬ�ڿ����м��Ⱥֱ����������Һ�У���ֽӴ���ͭƬ�������ٵ��ǣ�������
| A�� | HNO3 | B�� | ��ˮ�Ҵ� | C�� | ʯ��ˮ | D�� | ���� |
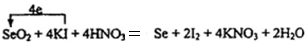 ��
��