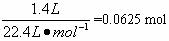��Ŀ����
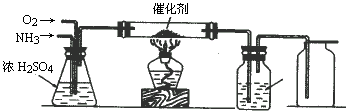
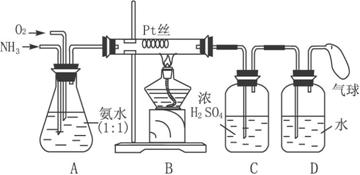
����ijѧ����Ƶ��ô���������ȡ����HNO3��Һ��ʵ��װ�ã�����ͼ��ʾ����ش��������⣺
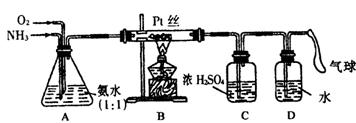
(1)ʵ�����Ʊ�NH3�����з�������ѡ�õ���__________(�����)��
�ٹ�̬�Ȼ������ʯ�һ�ϼ��� �ڹ�̬�Ȼ�識��ȷֽ�
����ʯ���еμ�Ũ��ˮ ���Ȼ����Һ������������Һ����
(2)װ��B�з�����Ӧ�Ļ�ѧ����ʽΪ__________����ʵ������У�����Pt˿���Ⱥ���ȥ�ƾ��ƣ�����pt˿���������ֺ��ȣ��ɴ˿��жϸ÷�Ӧ��__________��
(3)װ��C��������__________��װ��C�е�������__________��Ϊȷ��D�о����ܶ�����HNO3����ͨ��O2��NH3�������Ӧ����__________��
(4)���ڸ����´�����ʱ���и���Ӧ������4 NH3+3O2![]() 2 N2+6H2O��������װ���в�����������������D����1.0 mol��L��HNO3��Һ150 mL���������ռ��Ļ���������Ϊ1 400 mL(��״��)������NO2��O2��N2�������Ϊ2��2 �� 1����������NO�İ�ռ���������������Ϊ___________��
2 N2+6H2O��������װ���в�����������������D����1.0 mol��L��HNO3��Һ150 mL���������ռ��Ļ���������Ϊ1 400 mL(��״��)������NO2��O2��N2�������Ϊ2��2 �� 1����������NO�İ�ռ���������������Ϊ___________��
(1)�� ��
(2)4 NH3+5O2![]() 4NO+6H2O ���ȷ�Ӧ (3)���ջ�������е�NH3
4NO+6H2O ���ȷ�Ӧ (3)���ջ�������е�NH3
�����ݴ�ŨH2SO4��ð�����Ҽ���ƿ��ŨH2SO4�ϲ��ռ�ʺ���ɫ 2:1
(4)87.5����![]()

 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�