��Ŀ����
����Ŀ��������X��һ�����ϣ��ɲ�����ϩ��ױ�Ϊ��Ҫԭ�ϣ�������·�ߺϳɣ�
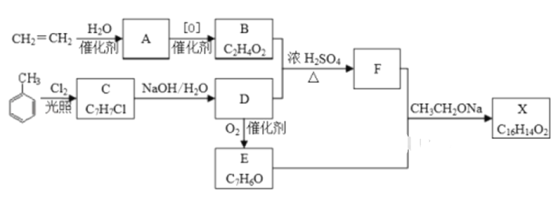
��֪��RX![]() ROH��RCHO+CH3COOR��
ROH��RCHO+CH3COOR��![]() RCH=CHCOOR��
RCH=CHCOOR��
��ش�
��1��C �Ľṹ��ʽ________________ ��A�й����ŵ�������__________
��2��B+D��F�Ļ�ѧ����ʽ___________________________���䷴Ӧ����Ϊ_____________
��3��X�Ľṹ��ʽ___________________
��4��D��E�Ļ�ѧ����ʽ____________________________________
��5��F�ж���ͬ���칹�壬��������������ͬ���칹����Ŀ��______�֣���д������һ��ͬ���칹��Ľṹ��ʽ________
���DZ��Ķ�Ԫȡ����
���ܷ���ˮ�⼰������Ӧ
�ۺ˴Ź���������5�����շ壬�����֮��Ϊ3��2��2��2��1
���𰸡�![]() �ǻ�
�ǻ� ![]() ȡ������������Ӧ
ȡ������������Ӧ ![]()
![]() 2
2 ![]() ��
�� ![]()
��������
���ݿ�ͼ֪CH2=CH2+H2O![]() A(CH3CH2OH)��BΪ
A(CH3CH2OH)��BΪ![]() CH3COOH;��
CH3COOH;�� ֪CΪ
֪CΪ![]() �� DΪ
�� DΪ![]() ��������ΪE.(
��������ΪE.(![]() )��B+D
)��B+D![]() FΪ
FΪ![]() ��E+F+
��E+F+![]() X(
X(![]() )��
)��
��1���ɿ�ͼ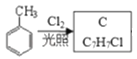 ֪C �Ľṹ��ʽΪ
֪C �Ľṹ��ʽΪ![]() ��A ΪCH3CH2OH�����еĹ����ŵ��������ǻ����𰸣�
��A ΪCH3CH2OH�����еĹ����ŵ��������ǻ����𰸣�![]() ���ǻ���
���ǻ���
��2��BΪCH3COOH ��DΪ![]() ����B+D��F�Ļ�ѧ����ʽ
����B+D��F�Ļ�ѧ����ʽ![]() ���䷴Ӧ����Ϊȡ����Ӧ���𰸣�
���䷴Ӧ����Ϊȡ����Ӧ���𰸣� ![]() ��ȡ������������Ӧ��
��ȡ������������Ӧ��
��3��������������֪X�Ľṹ��ʽ![]() ���𰸣�
���𰸣�![]() ��
��
��4��DΪ![]() ��D
��D![]() E,����D��E�Ļ�ѧ����ʽΪ��
E,����D��E�Ļ�ѧ����ʽΪ��![]() ��
��
��5����FΪ![]() ��F�ж���ͬ���칹�壬��������������ͬ���칹����Ŀ��
��F�ж���ͬ���칹�壬��������������ͬ���칹����Ŀ��![]() ��
��![]() ��2�֡��𰸣�
��2�֡��𰸣�![]() ��
��![]() ��
��


