��Ŀ����
(16��)��ѧ��ȤС���ijƷ��������Ħ�����ɷּ��京����������̽����
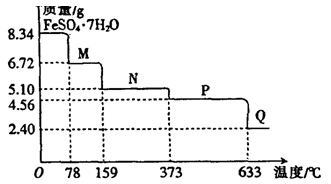
������ϣ�������Ħ������̼��ơ�����������ɣ������������ɷ���������ʱ���������ɡ�
I��Ħ���������������Ķ��Լ���
ȡ����������Ʒ����ˮ���衢���ˡ�����������ʵ��
��1��������ɱ����е���գ�
II��������Ʒ��̼��ƵĶ����ⶨ
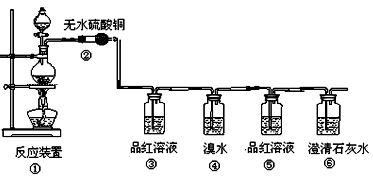
������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨC�����ɵ�BaCO3������������ȷ��̼��Ƶ�����������
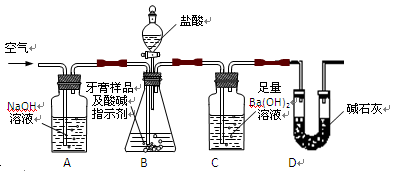
����ʵ����̻ش��������⣺
��2����ʵ��������Ⱥ����ι���������ڶ��ι��������Ŀ���ǣ�
������������������������������������������������������������ ��
��3����C�з�Ӧ����BaCO3�Ļ�ѧ����ʽ���������������������������� ��
��4�������и����ʩ�У�������߲ⶨȷ�ȵ��� ���� �����ţ���
A���ڼ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2����
B���μ�����˹���
C����A��B֮������ʢ��Ũ�����ϴ��װ��
D����B��C֮������ʢ�б���̼��������Һ��ϴ��װ��
��5����ʵ����ȷ��ȡ8.00g��Ʒ���ݣ��������βⶨ�����BaCO3ƽ������Ϊ3.94g������Ʒ��̼��Ƶ���������Ϊ ����
��6����ijͬѧ�����C�е�Ba(OH)2����ŨH2SO4��ͨ���ⶨDװ�÷�Ӧǰ���������Ҳ���ԲⶨCaCO3�ĺ��������跴ӦǰDװ�õ�����Ϊm1��ʵ�������Dװ�õ�����Ϊm2������Ʒ��CaCO3������Ϊ ������ʵ��֤�����˲ⶨ�Ľ��ƫ�ߣ�ԭ�������� ������
������ϣ�������Ħ������̼��ơ�����������ɣ������������ɷ���������ʱ���������ɡ�
I��Ħ���������������Ķ��Լ���
ȡ����������Ʒ����ˮ���衢���ˡ�����������ʵ��
��1��������ɱ����е���գ�
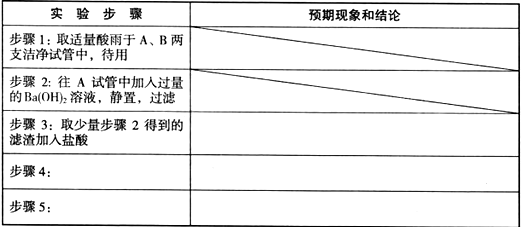
| ʵ�鲽�� | ʵ������ | ��Ӧ�����ӷ���ʽ |
| �������м������NaOH��Һ�� | | �������������� �������������� |
| ���ˣ���������Һ��ͨ�����������̼�� | ������������ ������ | �������������� �������������� |
| �����������ϡ���� | �������������� ���� | |
������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨC�����ɵ�BaCO3������������ȷ��̼��Ƶ�����������

����ʵ����̻ش��������⣺
��2����ʵ��������Ⱥ����ι���������ڶ��ι��������Ŀ���ǣ�
������������������������������������������������������������ ��
��3����C�з�Ӧ����BaCO3�Ļ�ѧ����ʽ���������������������������� ��
��4�������и����ʩ�У�������߲ⶨȷ�ȵ��� ���� �����ţ���
A���ڼ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2����
B���μ�����˹���
C����A��B֮������ʢ��Ũ�����ϴ��װ��
D����B��C֮������ʢ�б���̼��������Һ��ϴ��װ��
��5����ʵ����ȷ��ȡ8.00g��Ʒ���ݣ��������βⶨ�����BaCO3ƽ������Ϊ3.94g������Ʒ��̼��Ƶ���������Ϊ ����
��6����ijͬѧ�����C�е�Ba(OH)2����ŨH2SO4��ͨ���ⶨDװ�÷�Ӧǰ���������Ҳ���ԲⶨCaCO3�ĺ��������跴ӦǰDװ�õ�����Ϊm1��ʵ�������Dװ�õ�����Ϊm2������Ʒ��CaCO3������Ϊ ������ʵ��֤�����˲ⶨ�Ľ��ƫ�ߣ�ԭ�������� ������
(16��)��1������Al(OH)3+OH�D=AlO2�D +2H2O (1��)��
���а�ɫ��������(1��)
��AlO2�D+CO2+2H2O=Al(OH)3��+HCO3�D (1��)���ܳ����ܽ⣬���������(1��)
��2�������ϲ�����װ���е�CO2��ʹ���ɵ�CO2��ȫ������ (2��)
��3����CO2+Ba(OH)2 =BaCO3��+H2O (2��)
��4����CD (2��)
��5����0.25(25%) (2��)
��6����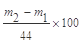 (2��) Dװ�����շ�Ӧ���ɵ�CO2�⣬�������ջӷ�����HCl��ͬʱ�����е�H2O��CO2Ҳ�����Dװ�á�ʹm2-m1ƫ��(2��)��
(2��) Dװ�����շ�Ӧ���ɵ�CO2�⣬�������ջӷ�����HCl��ͬʱ�����е�H2O��CO2Ҳ�����Dװ�á�ʹm2-m1ƫ��(2��)��
���а�ɫ��������(1��)
��AlO2�D+CO2+2H2O=Al(OH)3��+HCO3�D (1��)���ܳ����ܽ⣬���������(1��)
��2�������ϲ�����װ���е�CO2��ʹ���ɵ�CO2��ȫ������ (2��)
��3����CO2+Ba(OH)2 =BaCO3��+H2O (2��)
��4����CD (2��)
��5����0.25(25%) (2��)
��6����
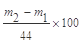 (2��) Dװ�����շ�Ӧ���ɵ�CO2�⣬�������ջӷ�����HCl��ͬʱ�����е�H2O��CO2Ҳ�����Dװ�á�ʹm2-m1ƫ��(2��)��
(2��) Dװ�����շ�Ӧ���ɵ�CO2�⣬�������ջӷ�����HCl��ͬʱ�����е�H2O��CO2Ҳ�����Dװ�á�ʹm2-m1ƫ��(2��)����1������������NaOH��Һ��Ӧ�����ӷ���ʽ��д��Ҫ���������Al(OH)3��OH����[Al(OH)4]����Al(OH)3����OH����AlO2����2H2O��2�����ɵ���NaAlO2��Һ��ͨ��CO2������Al(OH)3��ɫ�������ɣ���������NaHCO3������������CO2���������Al(OH)3�����ܽ⡣ѧ����������ʵ������Ҫ�Ƚ���ʵ�Ļ���������2��ʵ����������������ͨ������������ɵ�CO2ȫ������C�У�ʹ֮��ȫ��Ba(OH)2��Һ���ա���������ѧ�������Ӵ�����3��CO2��Ba(OH)2��BaCO3����H2O�������ķ���ʽ��д����4���ڼ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2���壬��Ӱ��������μ�����˹��죬��֤���ɵ�CO2��ȫ�����ա���A��B֮������ʢ��Ũ�����ϴ��װ�ã���Ϊ����װ���в���Ҫ�����ˮ�ݲ���Ӱ��CO2���ա���B��C֮������ʢ�б���̼��������Һ��ϴ��װ�ã�Cƿ��������Ba(OH)2����������CO2�е�HCl������Ӱ��CO2���գ����Բ���Ҫ��ȥCO2�е�HCl��ѡcd����5��BaCO3����Ϊ3.94g ��n(BaCO3)=0.0200mol�� ��n(CaCO3)=0.0200mol������Ϊ2.00g������Ʒ��̼��Ƶ���������Ϊ25%�����ʼ���Ƚϼ���6��Ba(OH)2��Һ��������ˮ�������Ȼ�������ȣ��������ƫ�����Կ��Իش�B�е�ˮ�������Ȼ�������Ƚ���װ��C�С�

��ϰ��ϵ�д�
�����Ŀ