��Ŀ����
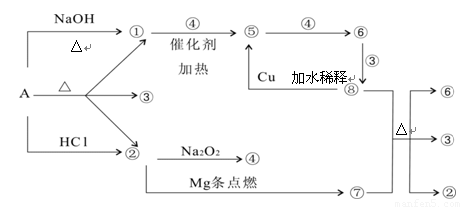
����A�ܷ�����ͼ��ʾ�ķ�Ӧ��ͼ�Т١���ֱ�����йط�Ӧ�е�һ�����ʣ�ijЩ��������ȥ�������Т١��ڡ��ܡ���Ϊ��ɫ���壮
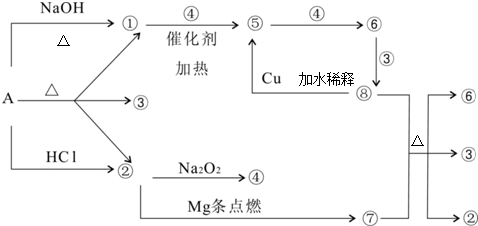
��������и��⣺
��1��д����ѧʽA
��2��д�����з�Ӧ����ʽ����+�ߡ���+��+�ޣ�
��+�ܡ��ݣ�
��3������ݵ����ӷ���ʽΪ��

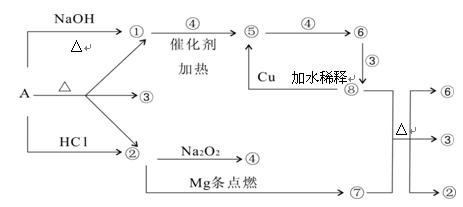
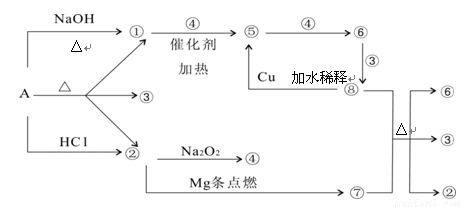
��������и��⣺
��1��д����ѧʽA
��NH4��2CO3
��NH4��2CO3
��NO2
NO2
��C
C
����2��д�����з�Ӧ����ʽ����+�ߡ���+��+�ޣ�
4HNO3��Ũ��+C
CO2��+4N02��+2H2O
| ||
4HNO3��Ũ��+C
CO2��+4N02��+2H2O
| ||
��+�ܡ��ݣ�
4NH3+5O2
4NO+6H2O
| ||
4NH3+5O2
4NO+6H2O
��
| ||
��3������ݵ����ӷ���ʽΪ��
3Cu+8H++2NO3-�T3Cu2++2NO+4H2O
3Cu+8H++2NO3-�T3Cu2++2NO+4H2O
����������ΪA�ڼ������������ɵ����壬��ΪNH3����ΪA���������������ɵ����壬����Na2O2��Mg��Ӧ��ӦΪCO2��������AӦΪ��NH4��2CO3����ΪH2O����ΪO2����ΪNO����ΪN02����ΪHNO3����ΪC��������ʵ����ʺ���ĿҪ��ɽ����⣮
����⣺��ΪA�ڼ������������ɵ����壬��ΪNH3����ΪA���������������ɵ����壬����Na2O2��Mg��Ӧ��ӦΪCO2��������AӦΪ��NH4��2CO3����ΪH2O����ΪO2����ΪNO����ΪN02����ΪHNO3����ΪC����
��1�������Ϸ�����֪AΪ��NH4��2CO3����ΪN02����ΪC���ʴ�Ϊ����NH4��2CO3��N02��C��
��2����+�ߡ���+��+�ķ�ӦΪŨ�����C�ķ�Ӧ������ʽΪ4HNO3��Ũ��+C
CO2��+4N02��+2H2O��
��+�ܡ��ݵķ�ӦΪ�����Ĵ�������Ӧ������ʽΪ4NH3+5O2
4NO+6H2O��
�ʴ�Ϊ��4HNO3��Ũ��+C
CO2��+4N02��+2H2O��4NH3+5O2
4NO+6H2O��
��3��ϡ�����Cu��Ӧ����������ͭ��NO����Ӧ�����ӷ���ʽΪ3Cu+8H++2NO3-�T3Cu2++2NO+4H2O��
�ʴ�Ϊ��3Cu+8H++2NO3-�T3Cu2++2NO+4H2O��
��1�������Ϸ�����֪AΪ��NH4��2CO3����ΪN02����ΪC���ʴ�Ϊ����NH4��2CO3��N02��C��
��2����+�ߡ���+��+�ķ�ӦΪŨ�����C�ķ�Ӧ������ʽΪ4HNO3��Ũ��+C
| ||
��+�ܡ��ݵķ�ӦΪ�����Ĵ�������Ӧ������ʽΪ4NH3+5O2
| ||
�ʴ�Ϊ��4HNO3��Ũ��+C
| ||
| ||
��3��ϡ�����Cu��Ӧ����������ͭ��NO����Ӧ�����ӷ���ʽΪ3Cu+8H++2NO3-�T3Cu2++2NO+4H2O��
�ʴ�Ϊ��3Cu+8H++2NO3-�T3Cu2++2NO+4H2O��
���������⿼��������ƶϣ���Ŀ�ѶȲ�����ע��������ʵ�������Ϊ�ƶ����ͻ�ƿڽ�����ʵ������ƶϣ�

��ϰ��ϵ�д�
 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
�����Ŀ
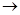 ��+��+�ޣ�
��+��+�ޣ�
 ��+��+�ޣ�
��+��+�ޣ�