��Ŀ����
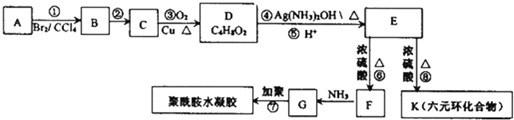
������ʳƷ����ը�������ʱ�����ı�ϩ������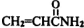 �������еȶ��ԣ�A-K�����л�����ת����ϵ��ͼ��ʾ�����������Ͳ�����ȥ������֪��AΪ��̬������״�����ܶ�Ϊ2.5g/L���Һ˴Ź���������4�����շ壻G�ȱ�ϩ������һ��CH2ԭ���ţ�
�������еȶ��ԣ�A-K�����л�����ת����ϵ��ͼ��ʾ�����������Ͳ�����ȥ������֪��AΪ��̬������״�����ܶ�Ϊ2.5g/L���Һ˴Ź���������4�����շ壻G�ȱ�ϩ������һ��CH2ԭ���ţ�
�밴Ҫ��ش��������⣺
��I��A�����ƣ� ����Ӧ�ٵķ�Ӧ���ͣ� ��B�Ľṹʽ�� ��K�������������ƣ� ����Ӧ�������Լ�����Ӧ������ ��
��2������B��NaOH�Ҵ���Һ���������·�������ȥ��Ӧ��ʵ�鷽��������Ӧ����������ͨ��ʢ�� ��ϴ��ƿ���ɹ۲쵽������Ϊ ��
��3��д�����л�ѧ����ʽ����Ӧ�ܣ� ����Ӧ�ࣺ ��
��4�����й��ڱ�ϩ������˵������ȷ���� �����ţ���
�����������ࣻ�����������ᷴӦ��������Ӧ������ʳϰ�ߣ���ʳ��ըʳƷ
��5����F������ͬ�����ŵ�ͬ���칹�壨��F���ж��֣���д�����з�ʽ�ṹ�Ľṹ��ʽ�� ��
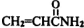 �������еȶ��ԣ�A-K�����л�����ת����ϵ��ͼ��ʾ�����������Ͳ�����ȥ������֪��AΪ��̬������״�����ܶ�Ϊ2.5g/L���Һ˴Ź���������4�����շ壻G�ȱ�ϩ������һ��CH2ԭ���ţ�
�������еȶ��ԣ�A-K�����л�����ת����ϵ��ͼ��ʾ�����������Ͳ�����ȥ������֪��AΪ��̬������״�����ܶ�Ϊ2.5g/L���Һ˴Ź���������4�����շ壻G�ȱ�ϩ������һ��CH2ԭ���ţ�
�밴Ҫ��ش��������⣺
��I��A�����ƣ�
��2������B��NaOH�Ҵ���Һ���������·�������ȥ��Ӧ��ʵ�鷽��������Ӧ����������ͨ��ʢ��
��3��д�����л�ѧ����ʽ����Ӧ�ܣ�
��4�����й��ڱ�ϩ������˵������ȷ����
�����������ࣻ�����������ᷴӦ��������Ӧ������ʳϰ�ߣ���ʳ��ըʳƷ
��5����F������ͬ�����ŵ�ͬ���칹�壨��F���ж��֣���д�����з�ʽ�ṹ�Ľṹ��ʽ��
���㣺�л�����ƶ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
������AΪ��̬������״�����ܶ�Ϊ2.5g/L������Է�������=2.5��22.4=56��������Cԭ�������Ŀ=
=4��8����A�ķ���ʽΪC4H8���Һ˴Ź���������4�����շ壬��AΪCH2=CHCH2CH3����BΪBrCH2CHBrCH2CH3��C����������Ӧ����D����֪B����������ˮ��Һ�����������·���ˮ�ⷴӦ����C����CΪHOCH2CH��OH��CH2CH3����D�ķ���ʽ��֪��C��ֻ��1��-OHת��Ϊ-CHO����DΪCH3CH2CH��OH��CHO��EΪCH3CH2CH��OH��COOH����Ԫ��������KΪ ��F�백����Ӧ�õ�G��G�ȱ�ϩ������һ��CH2ԭ���ţ���֪E������ȥ��Ӧ����F����FΪCH3CH=CHCOOH��GΪCH3CH=CHCOONH2��G�����Ӿ۷�Ӧ���Եõ�������ˮ�������ݴ˽��
��F�백����Ӧ�õ�G��G�ȱ�ϩ������һ��CH2ԭ���ţ���֪E������ȥ��Ӧ����F����FΪCH3CH=CHCOOH��GΪCH3CH=CHCOONH2��G�����Ӿ۷�Ӧ���Եõ�������ˮ�������ݴ˽��
| 56 |
| 12 |
 ��F�백����Ӧ�õ�G��G�ȱ�ϩ������һ��CH2ԭ���ţ���֪E������ȥ��Ӧ����F����FΪCH3CH=CHCOOH��GΪCH3CH=CHCOONH2��G�����Ӿ۷�Ӧ���Եõ�������ˮ�������ݴ˽��
��F�백����Ӧ�õ�G��G�ȱ�ϩ������һ��CH2ԭ���ţ���֪E������ȥ��Ӧ����F����FΪCH3CH=CHCOOH��GΪCH3CH=CHCOONH2��G�����Ӿ۷�Ӧ���Եõ�������ˮ�������ݴ˽�����
�⣺AΪ��̬������״�����ܶ�Ϊ2.5g/L������Է�������=2.5��22.4=56��������Cԭ�������Ŀ=
=4��8����A�ķ���ʽΪC4H8���Һ˴Ź���������4�����շ壬��AΪCH2=CHCH2CH3����BΪBrCH2CHBrCH2CH3��C����������Ӧ����D����֪B����������ˮ��Һ�����������·���ˮ�ⷴӦ����C����CΪHOCH2CH��OH��CH2CH3����D�ķ���ʽ��֪��C��ֻ��1��-OHת��Ϊ-CHO����DΪCH3CH2CH��OH��CHO��EΪCH3CH2CH��OH��COOH����Ԫ��������KΪ ��F�백����Ӧ�õ�G��G�ȱ�ϩ������һ��CH2ԭ���ţ���֪E������ȥ��Ӧ����F����FΪCH3CH=CHCOOH��GΪCH3CH=CHCOONH2��G�����Ӿ۷�Ӧ���Եõ�������ˮ������
��F�백����Ӧ�õ�G��G�ȱ�ϩ������һ��CH2ԭ���ţ���֪E������ȥ��Ӧ����F����FΪCH3CH=CHCOOH��GΪCH3CH=CHCOONH2��G�����Ӿ۷�Ӧ���Եõ�������ˮ������
��1��AΪCH2=CHCH2CH3������Ϊ��1-��ϩ����Ӧ����1-��ϩ���巢���ӳɷ�Ӧ��BΪBrCH2CHBrCH2CH3����ṹʽΪ�� ��KΪ
��KΪ ����������������Ϊ����������Ӧ�������Լ�����Ӧ��������������ˮ��Һ�����ȣ�
����������������Ϊ����������Ӧ�������Լ�����Ӧ��������������ˮ��Һ�����ȣ�
�ʴ�Ϊ��1-��ϩ���ӳɷ�Ӧ�� ����������������ˮ��Һ�����ȣ�
����������������ˮ��Һ�����ȣ�
��2������BrCH2CHBrCH2CH3��NaOH�Ҵ���Һ���������·�������ȥ��Ӧ��ʵ�鷽��������Ӧ����������ͨ��ʢ��������Ȼ�̼��Һ��ϴ��ƿ���ɹ۲쵽������Ϊ����Һ�Ⱥ�ɫ��ȥ��
�ʴ�Ϊ��������Ȼ�̼��Һ����Һ�Ⱥ�ɫ��ȥ��
��3����Ӧ�ܵĻ�ѧ����ʽΪ�� ��
��
��Ӧ��Ļ�ѧ����ʽΪ��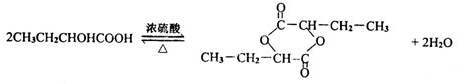 ��
��
�ʴ�Ϊ�� ��
��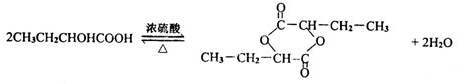 ��
��
��4���ٱ�ϩ��������O��NԪ�أ��������������࣬�ʴ���
�ں��м��Ի��Ű������������ᷴӦ������ȷ��
����ը�������ʱ�����ı�ϩ���������еȶ��ԣ�����Ӧ������ʳϰ�ߣ���ʳ��ըʳƷ������ȷ��
�ʴ�Ϊ���ڢۣ�
��5��FΪCH3CH=CHCOOH����F������ͬ�����ŵ�ͬ���칹�壨��F���ж��֣����з�ʽ�ṹ�Ľṹ��ʽ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
| 56 |
| 12 |
 ��F�백����Ӧ�õ�G��G�ȱ�ϩ������һ��CH2ԭ���ţ���֪E������ȥ��Ӧ����F����FΪCH3CH=CHCOOH��GΪCH3CH=CHCOONH2��G�����Ӿ۷�Ӧ���Եõ�������ˮ������
��F�백����Ӧ�õ�G��G�ȱ�ϩ������һ��CH2ԭ���ţ���֪E������ȥ��Ӧ����F����FΪCH3CH=CHCOOH��GΪCH3CH=CHCOONH2��G�����Ӿ۷�Ӧ���Եõ�������ˮ��������1��AΪCH2=CHCH2CH3������Ϊ��1-��ϩ����Ӧ����1-��ϩ���巢���ӳɷ�Ӧ��BΪBrCH2CHBrCH2CH3����ṹʽΪ��
 ��KΪ
��KΪ ����������������Ϊ����������Ӧ�������Լ�����Ӧ��������������ˮ��Һ�����ȣ�
����������������Ϊ����������Ӧ�������Լ�����Ӧ��������������ˮ��Һ�����ȣ��ʴ�Ϊ��1-��ϩ���ӳɷ�Ӧ��
 ����������������ˮ��Һ�����ȣ�
����������������ˮ��Һ�����ȣ���2������BrCH2CHBrCH2CH3��NaOH�Ҵ���Һ���������·�������ȥ��Ӧ��ʵ�鷽��������Ӧ����������ͨ��ʢ��������Ȼ�̼��Һ��ϴ��ƿ���ɹ۲쵽������Ϊ����Һ�Ⱥ�ɫ��ȥ��
�ʴ�Ϊ��������Ȼ�̼��Һ����Һ�Ⱥ�ɫ��ȥ��
��3����Ӧ�ܵĻ�ѧ����ʽΪ��
 ��
����Ӧ��Ļ�ѧ����ʽΪ��
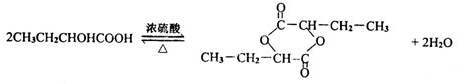 ��
���ʴ�Ϊ��
 ��
��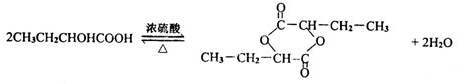 ��
����4���ٱ�ϩ��������O��NԪ�أ��������������࣬�ʴ���
�ں��м��Ի��Ű������������ᷴӦ������ȷ��
����ը�������ʱ�����ı�ϩ���������еȶ��ԣ�����Ӧ������ʳϰ�ߣ���ʳ��ըʳƷ������ȷ��
�ʴ�Ϊ���ڢۣ�
��5��FΪCH3CH=CHCOOH����F������ͬ�����ŵ�ͬ���칹�壨��F���ж��֣����з�ʽ�ṹ�Ľṹ��ʽ��
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
���������⿼���л����ƶϣ�����A����Է�������ȷ����ṹ���ٽ��ת����ϵ�����ƶϣ����չ����ŵ�������ת���ǹؼ����Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
����˵����ȷ���ǣ�������
| A��Ϊ�˽��ͳɱ����ù�ҵ�ƾ����Ҿ� |
| B����֬ˮ����Ƶ÷��� |
| C��ʹ��̫���ܵȴ��滯ʯȼ�ϣ����ϵ�̼���ܼ��ŵ�Ҫ�� |
| D������ϳ���ά����Ҫ�ɷ־�Ϊ��ά�� |
��NAΪ����ӵ���������ֵ������˵����ȷ���ǣ�������
| A��1L 1mol?L-1��Na2CO3 ��Һ�к���CO32-����ĿΪNA |
| B��25��ʱpH=13��NaOH��Һ�к���OH-����ĿΪ0.1NA |
| C��һ�������£�2.3g��Na��ȫ��O2��Ӧ����3.6g����ʱʧȥ�ĵ�����һ��Ϊ0.1NA |
| D��1mol Fe��������ϡHNO3��Ӧ��ת��2NA������ |