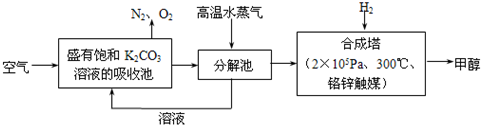
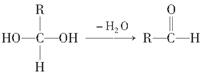
��Ŀ����
 ��2011?�㶫�����ù��ܺ�������ɽ�CO2��H2O��g��ת��ΪCH4��O2�����������ʱ���ڲ�ͬ������I��II��III�������£�CH4���������ʱ��ı仯��ͼ��ʾ��
��2011?�㶫�����ù��ܺ�������ɽ�CO2��H2O��g��ת��ΪCH4��O2�����������ʱ���ڲ�ͬ������I��II��III�������£�CH4���������ʱ��ı仯��ͼ��ʾ����1����0-30Сʱ�ڣ�CH4��ƽ����������V��V���V��Ӵ�С��˳��Ϊ
V��V��V��
V��V��V��
����Ӧ��ʼ���12Сʱ�ڣ��ڵ�
��
��
�ִ����������£��ռ���CH4��࣮��2��������CH4��H2O��g��ͨ��۽�̫���ܷ�Ӧ����������Ӧ��CH4��g��+H2O��g��?CO��g��+3H2��g�����÷�Ӧ�ġ�H=+206kJ?mol-1
���ڴ��������ͼ�У�������Ӧ��������ϵ�������仯ͼ�����б�Ҫ�ı�ע��
�ڽ������ʵ�����CH4��H2O��g������1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬ƽ�ⳣ��K=27����ʱ���CO�����ʵ���Ϊ0.10mol����CH4��ƽ��ת���ʣ�������������λ��Ч���֣�
��3����֪��CH4��g��+2O2��g���TCO2��g��+2H2O��g����H=-802kJ?mol-1
д����CO2����CO���Ȼ�ѧ����ʽ
CO2��g��+3H2O��g���T2O2��g��+CO��g��+3H2��g����H=+1008 kJ?mol-1
CO2��g��+3H2O��g���T2O2��g��+CO��g��+3H2��g����H=+1008 kJ?mol-1
����������1����ͬʱ���������ʵ����ı仯��Խ����ƽ������Խ����ͬʱ���������ʵ����ı仯��ԽС��ƽ����Ӧ����ԽС����ͼ2��֪��Ӧ��ʼ���12Сʱ�ڣ��ڵڢ��ִ����������£��ռ���CH4��ࣻ
��2����CH4��g��+H2O��g��?CO��g��+3H2��g�����÷�Ӧ�ġ�H=+206kJ?mol-1����Ӧ�����ȷ�Ӧ����Ӧ�������������������������������仯����ͼ��
�����ݺϳ�ƽ������ʽ��ʽ����õ�ת���ʣ�
��3�����ݸ�˹���ɺ��Ȼ�ѧ����ʽ����õ���
��2����CH4��g��+H2O��g��?CO��g��+3H2��g�����÷�Ӧ�ġ�H=+206kJ?mol-1����Ӧ�����ȷ�Ӧ����Ӧ�������������������������������仯����ͼ��
�����ݺϳ�ƽ������ʽ��ʽ����õ�ת���ʣ�
��3�����ݸ�˹���ɺ��Ȼ�ѧ����ʽ����õ���
����⣺��1����ͼ2��֪����0��30h�ڣ���������ʵ����仯��Ϊ��n������n������n��������0��30h�ڣ�CH4��ƽ����������v����v����v����
��ͼ2��֪��Ӧ��ʼ���12Сʱ�ڣ��ڵڢ��ִ����������£��ռ���CH4��ࣻ
�ʴ�Ϊ��V��V��V��
��2����CH4��g��+H2O��g��?CO��g��+3H2��g�����÷�Ӧ�ġ�H=+206kJ?mol-1����Ӧ�����ȷ�Ӧ����Ӧ��������ϵ�������仯ͼΪ�� ��
��
�ʴ�Ϊ��
�ڽ������ʵ�����CH4��H2O��g������1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬ƽ�ⳣ��K=27����ʱ���CO�����ʵ���Ϊ0.10mol������ƽ��������������CH4��ƽ��ת���ʣ�
CH4��g��+H2O��g��?CO��g��+3H2��g��
��ʼ����mol�� x x 0 0
�仯����mol�� 0.10 0.10 0.10 0.30
ƽ������mol�� x-0.10 x-0.10 0.10 0.30
K=
=
=27
����õ�x=0.11mol
�����ת����=
��100%=91%
�ʴ�Ϊ��91%
��3����CH4��g��+H2O��g��?CO��g��+3H2��g������H=+206kJ?mol-1
��CH4��g��+2O2��g���TCO2��g��+2H2O��g����H=-802kJ?mol-1
���ݸ�˹���ɢ�-�ڵõ���CO2��g��+3H2O��g���T2O2��g��+CO��g��+3H2��g����H=+1008 kJ?mol-1
�ʴ�Ϊ��CO2��g��+3H2O��g���T2O2��g��+CO��g��+3H2��g����H=+1008 kJ?mol-1
��ͼ2��֪��Ӧ��ʼ���12Сʱ�ڣ��ڵڢ��ִ����������£��ռ���CH4��ࣻ
�ʴ�Ϊ��V��V��V��
��2����CH4��g��+H2O��g��?CO��g��+3H2��g�����÷�Ӧ�ġ�H=+206kJ?mol-1����Ӧ�����ȷ�Ӧ����Ӧ��������ϵ�������仯ͼΪ��
 ��
���ʴ�Ϊ��

�ڽ������ʵ�����CH4��H2O��g������1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬ƽ�ⳣ��K=27����ʱ���CO�����ʵ���Ϊ0.10mol������ƽ��������������CH4��ƽ��ת���ʣ�
CH4��g��+H2O��g��?CO��g��+3H2��g��
��ʼ����mol�� x x 0 0
�仯����mol�� 0.10 0.10 0.10 0.30
ƽ������mol�� x-0.10 x-0.10 0.10 0.30
K=
| c(CO)c3(H2) |
| c(CH4)c(H2O) |
| 0.10��0.303 |
| (x-0.10)2 |
����õ�x=0.11mol
�����ת����=
| 0.10mol |
| 0��11mol |
�ʴ�Ϊ��91%
��3����CH4��g��+H2O��g��?CO��g��+3H2��g������H=+206kJ?mol-1
��CH4��g��+2O2��g���TCO2��g��+2H2O��g����H=-802kJ?mol-1
���ݸ�˹���ɢ�-�ڵõ���CO2��g��+3H2O��g���T2O2��g��+CO��g��+3H2��g����H=+1008 kJ?mol-1
�ʴ�Ϊ��CO2��g��+3H2O��g���T2O2��g��+CO��g��+3H2��g����H=+1008 kJ?mol-1
���������⿼����ͼ������ͻ�ͼ��ķ�����ƽ�����Ӧ�ã��Ȼ�ѧ����ʽ����дԭ���˹���ɵļ���Ӧ�ã���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
�����Ŀ