��Ŀ����
(14��)��1����1 L AlCl3��FeCl3�����Һ�м��뺬a mol NaOH����Һʱ�������ij������ɴ����ֵ����������NaOH��Һ��������ʼ�ܽ⣬��ǰ������NaOH�����ﵽb molʱ���������ټ��٣���ԭ��Һ��Fe3+�����ʵ���Ũ��Ϊ ��AlCl3�����ʵ��� ��
��2����֪��2Fe3++2I����=��2Fe2++ I2������2Fe2++Br2 =��2Fe3++2Br��
������1mol FeI2��1.5mol FeBr2����Һ��ͨ��2mol Cl2����ʱ��������������������
�������ٵ���Һ��ͨ��3mol Cl2�������������Ӷ�Ӧ����������ֱ����������� ��
(3������m g��м�뺬�� n gHNO3��������Һǡ����ȫ��Ӧ���� m : n =" 1" : 2.7�� �÷�Ӧ�Ļ�ѧ����ʽΪ __________________________________________________�����軹ԭ����ֻ��һ�֣���ֻ����һ���Σ�
������ n g HNO3��ϡ������Һǡ��ʹ5.6g������ȫ�ܽ⣬���� n/4 gHNO3����ԭ��NO����������ԭ����� n �ķ�ΧΪ_________________________
��ij������п�����ᷴӦʱ�����ʵ���֮��Ϊ2:5����ʱ����Ļ�ԭ������____________
��2����֪��2Fe3++2I����=��2Fe2++ I2������2Fe2++Br2 =��2Fe3++2Br��
������1mol FeI2��1.5mol FeBr2����Һ��ͨ��2mol Cl2����ʱ��������������������
�������ٵ���Һ��ͨ��3mol Cl2�������������Ӷ�Ӧ����������ֱ����������� ��
(3������m g��м�뺬�� n gHNO3��������Һǡ����ȫ��Ӧ���� m : n =" 1" : 2.7�� �÷�Ӧ�Ļ�ѧ����ʽΪ __________________________________________________�����軹ԭ����ֻ��һ�֣���ֻ����һ���Σ�
������ n g HNO3��ϡ������Һǡ��ʹ5.6g������ȫ�ܽ⣬���� n/4 gHNO3����ԭ��NO����������ԭ����� n �ķ�ΧΪ_________________________
��ij������п�����ᷴӦʱ�����ʵ���֮��Ϊ2:5����ʱ����Ļ�ԭ������____________
(14��)(1)��4a-3b ��/3��b-a (2) ��I- Fe2+ �� Fe3+ I2 Br2
��3���� 5Fe +12HNO3��5Fe(NO3)2 + N2�� + 6H2O ��16.8 �� n �� 25.2 ��N2O��NH4NO3
��3���� 5Fe +12HNO3��5Fe(NO3)2 + N2�� + 6H2O ��16.8 �� n �� 25.2 ��N2O��NH4NO3
�����������1����AlCl3��FeCl3�����ʵ����ֱ���x��y��������йط�Ӧ�Ļ�ѧ����ʽ��AlCl3��3NaOH=Al(OH)3����3NaCl��FeCl3��3NaOH=Fe(OH)3����3NaCl��Al(OH)3��NaOH=NaAlO2��2H2O��֪��3x��3y��amol��x��bmol��amol�����y��
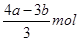 �����������ӵ�Ũ����
�����������ӵ�Ũ����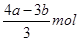 /L��
/L����2���ٸ���������ԭ��Ӧ�л�ԭ���Ļ�ԭ��ǿ�ڻ�ԭ����Ŀ�֪����ԭ��ǿ��˳����I����Fe2����Br����2mol�����õ�4mol���ӣ�1mol FeI2ʧȥ3mol������������������1mol�������ӡ�
��3mol�����õ�6mol���ӣ���Һ�е���������2mol���ӣ���������ʧȥ2.5mol�����Ի�������1.5mol�����ӣ�������������Fe3+��I2��Br2��
��3����������������ʵ���֮����
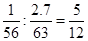 ��������������Ӧ����������������Ӧ�Ļ�ѧ����ʽ��5Fe +12HNO3��5Fe(NO3)2 + N2�� + 6H2O��
��������������Ӧ����������������Ӧ�Ļ�ѧ����ʽ��5Fe +12HNO3��5Fe(NO3)2 + N2�� + 6H2O�������������Ӧ�Ļ�ѧ����ʽ������Fe��4HNO3��Fe(NO3)3��NO����2H2O��3Fe��8HNO3��3Fe(NO3)2��2NO����4H2O�����ڷ�Ӧ����1/4�����ᱻ��ԭ�����Ը�������0.1mol��֪���������Сֵ�����ֵ�ֱ���
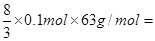 16.8g��
16.8g��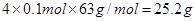 ����n��ȡֵ��Χ��16.8 �� n �� 25.2��
����n��ȡֵ��Χ��16.8 �� n �� 25.2������п�����ʵ�����2xmol����������4xmol����������п��2xmol������û�б���ԭ��������4xmol����ԭ��������5xmol��4xmol��xmol�����ݵ��ӵĵ�ʧ�غ��֪�������ڷ�Ӧ�еõ�4�����ӣ�����ԭ������N2O���������û�б���ԭ�����Ტû��ȫ����п���ӽ����������п����Ҳ������������泥���ʱ��Ӧ�Ļ�ѧ����ʽ����4Zn��10HNO3=4Zn(NO3)2��NH4NO3��3H2O�������ȷ�Ĵ���N2O��NH4NO3��
�����������ѶȽϴ��ؿ���ѧ�����������ۺ����ʣ���ѧ���ļ�����������˸��ߵ�Ҫ��������������ѧ���Ͻ�����˼ά��������ɢ˼ά�����Լ����Ӧ�����������������ѧ����ѧϰЧ�ʺ�Ӧ�������Լ��������⡢����������������ǿѧ����ѧϰ�����ġ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
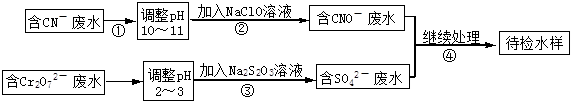 �ش��������⣺
�ش��������⣺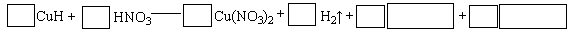
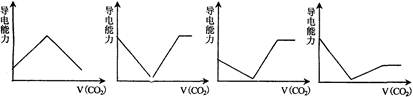
 ��ˮ��Һ�У�����������
��ˮ��Һ�У����������� ���壬��ַ�Ӧ���ټ��������ϡ���ᣬ����������Ŀû�б仯���ǣ� ��
���壬��ַ�Ӧ���ټ��������ϡ���ᣬ����������Ŀû�б仯���ǣ� ��


