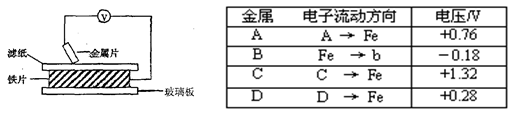
��Ŀ����
���ྻ�Ľ���ƬA��B��C��D�ֱ�����ڽ�������Һ����ֽ���沢ѹ������ͼ��ʾ������ÿ��ʵ��ʱ����¼ͼ�е�ѹ��ָ����ƶ�����͵�ѹ���Ķ������£�
��֪�������缫�Ľ�����������������Խ��ѹ���Ķ���Խ������ݱ������ݻش��������⣮
��1��______������������ǿ�Ļ�ԭ����______����һ�����ܴ�����ͭ��Һ���û���ͭ��
��2������ֽ��������Һ���������NaOH��Һ����������ֽ���ܿ�������ɫ���������Ľ�����______������ĸ�������Ӧ��صĵ缫��ӦʽΪ��������______�� ������______��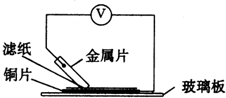
| ���� | ������������ | ��ѹ/V |
| A | A��Cu | +0.78 |
| B | Cu��B | -0.15 |
| C | C��Cu | +1.35 |
| D | D��Cu | +0.30 |
��1��______������������ǿ�Ļ�ԭ����______����һ�����ܴ�����ͭ��Һ���û���ͭ��
��2������ֽ��������Һ���������NaOH��Һ����������ֽ���ܿ�������ɫ���������Ľ�����______������ĸ�������Ӧ��صĵ缫��ӦʽΪ��������______�� ������______��

A-Cu����ʱ�����Ӵ�A��Cu������A�Ľ����Դ���ͭ��
B--Cu����ʱ�����Ӵ�Cu��B������ͭ�Ľ����Դ���B��
C--Cu����ʱ�����Ӵ�C��Cu������C�Ľ����Դ���ͭ��
D--Cu����ʱ�����Ӵ�D��Cu������D�Ľ����Դ���ͭ��
��1������������Ϣ��ԭ���ԭ��������������һ����ԭ��صĸ���������Ի��õĽ���������A��C��D����Cu���ã�������Բ�ֵԽ��ѹ����ʾ��Խ������C����ã��������Ա�Cu�����B���ʴ�Ϊ��C��B��
��2����ֽ��Ϊ��NaOH��Һ������������ɫ����[Cu��OH��2]��˵��Cu����������B��������
������ӦΪ��2Cu+4OH--4e-�T2Cu��OH��2����������ӦΪ��2H2O+O2+4e-�T4OH-��
�ʴ�Ϊ��B��2Cu+4OH--4e-�T2Cu��OH��2����2H2O+O2+4e-�T4OH-��
B--Cu����ʱ�����Ӵ�Cu��B������ͭ�Ľ����Դ���B��
C--Cu����ʱ�����Ӵ�C��Cu������C�Ľ����Դ���ͭ��
D--Cu����ʱ�����Ӵ�D��Cu������D�Ľ����Դ���ͭ��
��1������������Ϣ��ԭ���ԭ��������������һ����ԭ��صĸ���������Ի��õĽ���������A��C��D����Cu���ã�������Բ�ֵԽ��ѹ����ʾ��Խ������C����ã��������Ա�Cu�����B���ʴ�Ϊ��C��B��
��2����ֽ��Ϊ��NaOH��Һ������������ɫ����[Cu��OH��2]��˵��Cu����������B��������
������ӦΪ��2Cu+4OH--4e-�T2Cu��OH��2����������ӦΪ��2H2O+O2+4e-�T4OH-��
�ʴ�Ϊ��B��2Cu+4OH--4e-�T2Cu��OH��2����2H2O+O2+4e-�T4OH-��

��ϰ��ϵ�д�
�����Ŀ
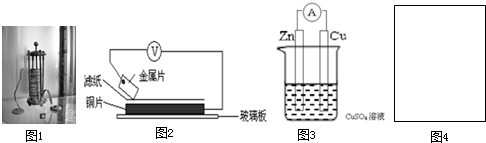
 ��������Ӧ��������
��������Ӧ�������� ���������ߣ���
���������ߣ��� �������缫����
�������缫���� ������С���ݣ����Ҳ��ͼ4���ڻ���װ��ͼ��ָ���缫���Ϻ͵������Һ�������Դ����������
������С���ݣ����Ҳ��ͼ4���ڻ���װ��ͼ��ָ���缫���Ϻ͵������Һ�������Դ����������
 ���ྻ�Ľ���ƬA��B��C��D�ֱ�����ڽ�������Һ����ֽ���沢ѹ������ͼ��ʾ������ÿ��ʵ��ʱ����¼ͼ�е�ѹ��ָ����ƶ�����͵�ѹ���Ķ������£�
���ྻ�Ľ���ƬA��B��C��D�ֱ�����ڽ�������Һ����ֽ���沢ѹ������ͼ��ʾ������ÿ��ʵ��ʱ����¼ͼ�е�ѹ��ָ����ƶ�����͵�ѹ���Ķ������£�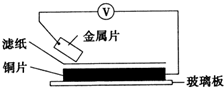 ���ྻ�Ľ���ƬA��B��C��D�ֱ�����ڽ���ij������Һ����ֽ���沢ѹ������ͼ��ʾ������ÿ��ʵ��ʱ����¼��ѹָ����ƶ�����͵�ѹ���Ķ������£�
���ྻ�Ľ���ƬA��B��C��D�ֱ�����ڽ���ij������Һ����ֽ���沢ѹ������ͼ��ʾ������ÿ��ʵ��ʱ����¼��ѹָ����ƶ�����͵�ѹ���Ķ������£�