��Ŀ����
���л�ѧ������д��ȷ����
A��NaHSˮ�ⷴӦ��HS��+ H2O   H3O++ S2�� H3O++ S2�� |
| B������������ˮ�������õ�ԭ��Al3++ 3H2O��Al(OH)3(����) + 3H+ |
| C����֪�� 2Zn��s��+O2��g��=2ZnO��s������H=��701.0kJ��mol-1 2Hg��l��+O2��g��=2HgO��s������H=��181.6kJ��mol-1 ��ӦZn��s��+ HgO��s��=ZnO��s��+ Hg��l������H=��259.7kJ��mol-1 |
| D��̼��������Һ�м������Ba(OH)2��Һ��2HCO3�� + Ba2+ + 2OH��= BaCO3��+ CO32��+ 2H2O |
C
���������A����ȷ���÷�Ӧ�ǵ��뷽��ʽ��ˮ�ⷽ��ʽӦ����HS����H2O
 OH����H2S��B����ȷ��Ӧ���ÿ�������ӣ����ݸ�˹���ɿ�֪��ѡ��C��ȷ��D����ȷ������������������������̼�ᱵ���������ƣ�������ȷ�Ĵ�ѡC��
OH����H2S��B����ȷ��Ӧ���ÿ�������ӣ����ݸ�˹���ɿ�֪��ѡ��C��ȷ��D����ȷ������������������������̼�ᱵ���������ƣ�������ȷ�Ĵ�ѡC���������������״�ѡA�����ֵ��뷽��ʽ��ˮ�ⷽ��ʽ�Ĺؼ��ǿ����������ɣ���Ϊ�����������ṩ���ӵģ�������������������ԭ�ӵĸ����Ǽ��ٵģ���ˮ���ǽ�������ӻ�OH���ģ����ԭ�ӵĸ��������ӵģ��ݴ˿��Խ����жϡ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2�� ���Ա�ʾ12Cԭ�ӻ�13Cԭ��
���Ա�ʾ12Cԭ�ӻ�13Cԭ�� ���Ա�ʾCO2���ӻ�SiO2����
���Ա�ʾCO2���ӻ�SiO2����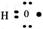 ���Ա�ʾ�ǻ�������������
���Ա�ʾ�ǻ�������������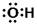
 HCl+HClO
HCl+HClO