��Ŀ����
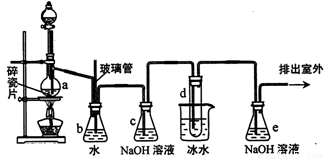
1��2һ����������������������Ӽ�����ͼΪʵ�����Ʊ�1��2һ���������װ��ͼ��ͼ�з�Һ©������ƿa�зֱ�װ��ŨH2SO4����ˮ�Ҵ����Թ�d��װ��Һ�塣
��֪��
��������б����£�

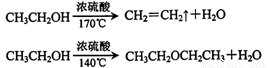
��1��ʵ����ӦѸ�ٽ��¶����ߵ�170�����ҵ�ԭ���� ��
��2����ȫƿb��ʵ�����ж������á���һ���Լ��ʵ������е���d�Ƿ�����������д����������ʱƿb�е����� ��
���ʵ���е���d����������Ϊ���ܵ�ԭ���� ��
��ȫƿb�������������� ��
��3������c��e�ж�ʢ��NaOH��Һ��c��NaOH��Һ�������� ��
��4��ijѧ��������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ����������������³������࣬���װ�õ�������û�����⣬�Է������ܵ�ԭ�� ����д���������ɣ�
��5����ȥ����������δ��Ӧ��Br2�����е���Ҫ����Ϊ ��Ҫ��һ���ᴿ�����в����б������ ��������ȷѡ��ǰ����ĸ����
A���ؽᾧ B������ C����ȡ D������
��6��ʵ����Ҳ���Գ�ȥdװ����ʢ��ˮ���ձ�����Ϊ����ˮֱ�Ӽ��뵽dװ�õ��Թ��ڣ����ʱ��ˮ����������ȴ1,2һ��������������⣬�������������� ��
��1�����ٸ������������ɣ�1�֣�
��2��b�г�ֱ����������һ��Һ��������1�֣�
������ȴ����Ʒ1 , 2������������װ��d�����̣�1�֣� ��ֹ������1�֣�
��3��������ϩ�����к��е�CO2��SO2���������壨2�֣�
��4����Ũ���Ὣ�����Ҵ����� �ڷ�������Ӧ�������� ���Ҵ��ӷ�
����ϩ���ٹ��죬δ��ȫ�����ӳɷ�Ӧ��2�֣�
��5�����ѣ�2�֣� D��2�֣�
��6��Һ��Br2��1 , 2���������飨2�֣�
��������
�����������1���Ҵ���Ũ����140��������£�������������ˮ���������ѣ�ʵ����ӦѸ�ٽ��¶����ߵ�170�����ҵ�ԭ���Ǽ��ٸ������������ɡ�
��2�����ݴ���ѹǿԭ�����Թ�d��������ʱ��b��ѹǿ��������ᵼ��b��ˮ���½����������е�ˮ������������������ʴ�Ϊ��b�г�ֱ����������һ��Һ�������� 1��2-������������̵�9��ϵͣ����ܹ�����ȴ��������ȴ���ͻ���װ��d��������ɶ�����bװ�õ��ܶ̽����������Է�ֹ������
��3������Ũ�������ǿ�����ԣ��ܹ�����̼��ͬʱ����ԭ�ɾ��д̼�����ζ�Ķ����������壬�����ϩ�����л���CO2��SO2���������壬����c��NaOH��Һ������������ϩ�����к��е�CO2��SO2����������
��4������ȫ����ɫʱ���������Ҵ���Ũ������Һ����������������³��������ԭ���������ϩ��������ͨ��Һ�壩�ٶȹ��죬���´���ϩû�к��巢����Ӧ�����⣬ʵ������У��Ҵ���Ũ����Ļ��Һû��Ѹ�ٴﵽ170�棨�𡰿��²�������ɣ����ᵼ�¸���Ӧ�ķ�������������ɡ�
��5��1��2-�������������ѵķе㲻ͬ�����߾�Ϊ�л�����ܣ�������ķ��������Ƿ��룬
��6�����ӷ������Թ�d�м���������ˮ����ˮ������ã����ɽ����¶ȣ����ٻӷ���
���㣺���鿼�����Ҵ��Ʊ�1��2-�������飬����������ʵĻ�����ѧ���ʡ�

 53���ò�ϵ�д�
53���ò�ϵ�д�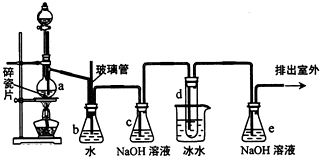 1��2һ����������������������Ӽ�����ͼΪʵ�����Ʊ�1��2һ���������װ��ͼ��ͼ�з�Һ©������ƿa�зֱ�װ��ŨH2SO4����ˮ�Ҵ����Թ�d��װ��Һ�壮
1��2һ����������������������Ӽ�����ͼΪʵ�����Ʊ�1��2һ���������װ��ͼ��ͼ�з�Һ©������ƿa�зֱ�װ��ŨH2SO4����ˮ�Ҵ����Թ�d��װ��Һ�壮��֪��CH3CH2OH
| Ũ���� |
| 170�� |
CH3CH2OH
| Ũ���� |
| 140�� |
��������б����£�
| �Ҵ� | 1��2-�������� | ���� | �� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� | ����ɫҺ�� |
| �ܶ�/g��cm-3 | 0.79 | 2.18 | 0.71 | 3.10 |
| �е�/�� | 78.5 | 131.4 | 34.6 | 58.8 |
| �۵�/�� | -114.3 | 9.79 | -116.2 | -7.2 |
| ˮ���� | ���� | ���� | �� | ���� |
��2����ȫƿb��ʵ�����ж������ã���һ���Լ��ʵ������е���d�Ƿ�����������д����������ʱƿb�е��������ʵ���е���d����������Ϊ���ܵ�ԭ���ǣ���ȫƿb��������������
��3������c��e�ж�ʢ��NaOH��Һ��c��NaOH��Һ��������
��4��ijѧ��������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ����������������³������࣬���װ�õ�������û�����⣬�Է������ܵ�ԭ��
��5����ȥ����������δ��Ӧ��Br2�����е���Ҫ����Ϊ
��6��ʵ����Ҳ���Գ�ȥdװ����ʢ��ˮ���ձ�����Ϊ����ˮֱ�Ӽ��뵽dװ�õ��Թ��ڣ����ʱ��ˮ����������ȴ1��2һ��������������⣬��������������