题目内容
(12分)【化学——化学与技术】
资源开发、材料制备及工农业生产等都离不开化学。请回答下列问题:
(1)工业制肥皂时,在皂化反应结束后需要在混合物中加入饱和食盐水。加入饱和食盐水的目的是 。
(2)Al2O3的熔点高达2050oC,工业上为了降低能量消耗,在金属铝的冶炼中通常采取的措施是 。
(3)工业上合成氨所需的氮气来源于 ,氢气来源于 ,写出工业上在催化剂作用下制取氢气的其中一个化学方程式 。
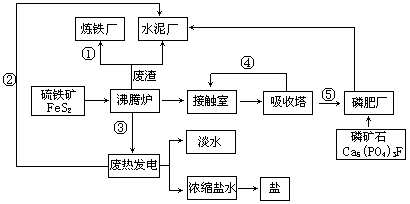
(4)工业制硫酸时,SO3的生成是在 (填设备名称)中进行的,工业上常采用浓硫酸吸收SO3,而不直接用水吸收的原因是 。在吸收塔中,为提高SO3的吸收率所采取的措施为 。
资源开发、材料制备及工农业生产等都离不开化学。请回答下列问题:
(1)工业制肥皂时,在皂化反应结束后需要在混合物中加入饱和食盐水。加入饱和食盐水的目的是 。
(2)Al2O3的熔点高达2050oC,工业上为了降低能量消耗,在金属铝的冶炼中通常采取的措施是 。
(3)工业上合成氨所需的氮气来源于 ,氢气来源于 ,写出工业上在催化剂作用下制取氢气的其中一个化学方程式 。
(4)工业制硫酸时,SO3的生成是在 (填设备名称)中进行的,工业上常采用浓硫酸吸收SO3,而不直接用水吸收的原因是 。在吸收塔中,为提高SO3的吸收率所采取的措施为 。
(1)盐析,使肥皂从混合液中分离出来。(2)在氧化铝中加入冰晶石,降低混合物的熔点。(3)空气,水和碳氢化合物,CH4+H2O CO+H2或CH4+2H2O
CO+H2或CH4+2H2O CO2+2H2。(4)接触室,防止形成酸雾,影响SO3的吸收效率。从塔的下部通入SO3,从塔上部淋洒浓H2SO4,SO3和浓H2SO4在填料表面接触而被吸收。
CO2+2H2。(4)接触室,防止形成酸雾,影响SO3的吸收效率。从塔的下部通入SO3,从塔上部淋洒浓H2SO4,SO3和浓H2SO4在填料表面接触而被吸收。
 CO+H2或CH4+2H2O
CO+H2或CH4+2H2O CO2+2H2。(4)接触室,防止形成酸雾,影响SO3的吸收效率。从塔的下部通入SO3,从塔上部淋洒浓H2SO4,SO3和浓H2SO4在填料表面接触而被吸收。
CO2+2H2。(4)接触室,防止形成酸雾,影响SO3的吸收效率。从塔的下部通入SO3,从塔上部淋洒浓H2SO4,SO3和浓H2SO4在填料表面接触而被吸收。试题分析: (1)工业制肥皂时,加入饱和食盐水的目的是盐析,使肥皂从混合液中分离出来。(2)Al2O3的熔点高达2050oC,工业上为了降低能量消耗,在金属铝的冶炼中通常采取的措施是在氧化铝中加入冰晶石,降低混合物的熔点。(3)工业上合成氨所需的氮气来源于空气,氢气来源于水和碳氢化合物,工业上在催化剂作用下制取氢气的化学方程式为CH4+H2O
 CO+H2或CH4+2H2O
CO+H2或CH4+2H2O CO2+2H2。(4)工业制硫酸时,SO3的生成是在接触室中进行的,工业上常采用浓硫酸吸收SO3,而不直接用水吸收的原因是防止形成酸雾,影响SO3的吸收效率。在吸收塔中,为提高SO3的吸收率所采取的措施为从塔的下部通入SO3,从塔上部淋洒浓H2SO4,SO3和浓H2SO4在填料表面接触而被吸收。
CO2+2H2。(4)工业制硫酸时,SO3的生成是在接触室中进行的,工业上常采用浓硫酸吸收SO3,而不直接用水吸收的原因是防止形成酸雾,影响SO3的吸收效率。在吸收塔中,为提高SO3的吸收率所采取的措施为从塔的下部通入SO3,从塔上部淋洒浓H2SO4,SO3和浓H2SO4在填料表面接触而被吸收。
练习册系列答案
 课堂全解字词句段篇章系列答案
课堂全解字词句段篇章系列答案 步步高口算题卡系列答案
步步高口算题卡系列答案
相关题目
 2Hg+O2↑
2Hg+O2↑ 3Fe+4CO2
3Fe+4CO2 2Mg+O2↑
2Mg+O2↑