��Ŀ����
��6�֣���1����ӡˢ��·ʱ�����Ȼ�����Һ��Ϊ����ʴҺ����ͭ���Ȼ�����Һ��ʴ�ķ���ʽΪ��B2FeCl3+Cu=2FeCl2+CuCl2���Ȼ�����ҺҲ��������Ӧ��2FeCl3+Fe=3FeCl2������ʢ���Ȼ�����Һ���ձ���ͬʱ�������ۺ�ͭ�ۣ���Ӧ�������ձ��ײ����ܳ��ֵ������ ����ѡ��
���ͭ���Ȼ�����Һ��ʴ�Ļ�ѧ����ʽ��дΪ���ӷ���ʽ��
��
��2��ij�ӵ������м��������������ŷŵĹ�ҵ��ˮ�У�������K+��Ag+��Fe3+��C1��?��OH����NO3���������ӡ�����ü׳��ķ�ˮ���Գʼ��ԣ������֪�ҳ���ˮ������������������ ��
| A����ͭ���� | B��������ͭ | C��������ͭ | D��������ͭ |
��
��2��ij�ӵ������м��������������ŷŵĹ�ҵ��ˮ�У�������K+��Ag+��Fe3+��C1��?��OH����NO3���������ӡ�����ü׳��ķ�ˮ���Գʼ��ԣ������֪�ҳ���ˮ������������������ ��
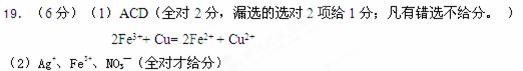
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 2��6 mol H2�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
2��6 mol H2�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
 __��
__��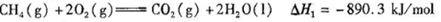

 ����ȾΪ�弶�ض���Ⱦ������Ϊ�ó��п�����Ⱦ����Ҫ��Ⱦ����
����ȾΪ�弶�ض���Ⱦ������Ϊ�ó��п�����Ⱦ����Ҫ��Ⱦ����