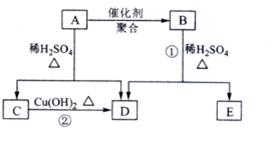
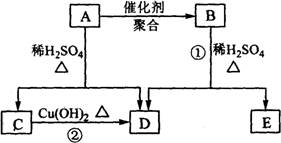
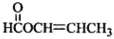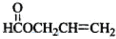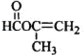��Ŀ����
������A��Է�������Ϊ86��̼����������Ϊ55.8%����Ϊ7.0%������Ϊ����A����ط�Ӧ��ͼ��ʾ����֪R-CH=CHOH��ϩ�������ȶ����ܿ�ת��ΪR-CH2CHO��
����������Ϣ�ش��������⣺
��1��A�ķ���ʽΪ ��
��2����Ӧ�ڵĻ�ѧ����ʽ�� ��
��3��A�Ľṹ��ʽ�� ��
��4����Ӧ�ٵĻ�ѧ����ʽ�� ��
��5��A�ж���ͬ���칹�壬д���ĸ�ͬʱ���㣨i���ܷ���ˮ�ⷴӦ��ii����ʹ������Ȼ�̼��Һ��ɫ����������ͬ���칹��Ľṹ��ʽ�� �� �� �� ��
��6��A����һ��ͬ���칹�壬�����������̼ԭ����һ��ֱ���ϣ����Ľṹ��ʽΪ ��

���𰸡�������������A��Է�������Ϊ86��̼����������Ϊ55.8%����Ϊ7.0%������Ϊ����A��C��H��Oԭ�ӵ�ԭ�Ӹ���֮��= ��
�� =2��3��1�����A����Է�������֪��A�ķ���ʽΪC4H6O2��A�ܷ����ۺϷ�Ӧ����B��˵��A�к���̼̼˫����A��ˮ������C��D��C�ܺ�����������ͭ��Ӧ����D��˵��C�к���ȩ����AΪ������C��D��̼ԭ�Ӹ�����ͬ������C����ȩ��D�����ᣬ��ΪR-CH=CHOH��ϩ�������ȶ����ܿ�ת��ΪR-CH2CHO������A�Ľṹ��ʽΪCH3COOCH=CH2��A�����ۺϷ�Ӧ����B������B�Ľṹ��ʽΪ��
=2��3��1�����A����Է�������֪��A�ķ���ʽΪC4H6O2��A�ܷ����ۺϷ�Ӧ����B��˵��A�к���̼̼˫����A��ˮ������C��D��C�ܺ�����������ͭ��Ӧ����D��˵��C�к���ȩ����AΪ������C��D��̼ԭ�Ӹ�����ͬ������C����ȩ��D�����ᣬ��ΪR-CH=CHOH��ϩ�������ȶ����ܿ�ת��ΪR-CH2CHO������A�Ľṹ��ʽΪCH3COOCH=CH2��A�����ۺϷ�Ӧ����B������B�Ľṹ��ʽΪ��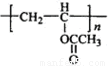 ��Bˮ�����������E����ϩ����E�Ľṹ��ʽΪ��
��Bˮ�����������E����ϩ����E�Ľṹ��ʽΪ�� ��
��
����⣺������A��Է�������Ϊ86��̼����������Ϊ55.8%����Ϊ7.0%������Ϊ����A��C��H��Oԭ�ӵ�ԭ�Ӹ���֮��=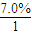 ��
��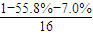 =2��3��1�����A����Է�������֪��A�ķ���ʽΪC4H6O2��A�ܷ����ۺϷ�Ӧ����B��˵��A�к���̼̼˫����A��ˮ������C��D��C�ܺ�����������ͭ��Ӧ����D��˵��C�к���ȩ����AΪ������C��D��̼ԭ�Ӹ�����ͬ������C����ȩ��D�����ᣬ��ΪR-CH=CHOH��ϩ�������ȶ����ܿ�ת��ΪR-CH2CHO������A�Ľṹ��ʽΪCH3COOCH=CH2��A�����ۺϷ�Ӧ����B������B�Ľṹ��ʽΪ��
=2��3��1�����A����Է�������֪��A�ķ���ʽΪC4H6O2��A�ܷ����ۺϷ�Ӧ����B��˵��A�к���̼̼˫����A��ˮ������C��D��C�ܺ�����������ͭ��Ӧ����D��˵��C�к���ȩ����AΪ������C��D��̼ԭ�Ӹ�����ͬ������C����ȩ��D�����ᣬ��ΪR-CH=CHOH��ϩ�������ȶ����ܿ�ת��ΪR-CH2CHO������A�Ľṹ��ʽΪCH3COOCH=CH2��A�����ۺϷ�Ӧ����B������B�Ľṹ��ʽΪ�� ��Bˮ�����������E����ϩ����E�Ľṹ��ʽΪ��
��Bˮ�����������E����ϩ����E�Ľṹ��ʽΪ�� ��
��
��1��ͨ�����Ϸ���֪��A�ķ���ʽC4H6O2���ʴ�Ϊ��C4H6O2��
��2��C����ȩ����ȩ��������ͭ��Ӧ�������ᡢ������ͭ��ˮ����Ӧ����ʽΪ��CH3CHO+2Cu��OH��2 CH3COOH+Cu2O+2H2O��
CH3COOH+Cu2O+2H2O��
�ʴ�Ϊ��CH3CHO+2Cu��OH��2 CH3COOH+Cu2O+2H2O��
CH3COOH+Cu2O+2H2O��
��3��ͨ�����Ϸ���֪��A�Ľṹ��ʽΪ��CH3COOCH=CH2���ʴ�Ϊ��CH3COOCH=CH2��
��4��B�Ľṹ��ʽΪ�� ��Bˮ�����������E����ϩ������Ӧ����ʽΪ��
��Bˮ�����������E����ϩ������Ӧ����ʽΪ��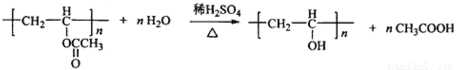 ��
��
�ʴ�Ϊ��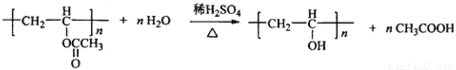 ��
��
��5��A�ж���ͬ���칹�壬д������ͬʱ���㣨i���ܷ���ˮ�ⷴӦ��˵��������������ii����ʹ������Ȼ�̼��Һ��ɫ��˵������̼̼˫���������������������A��ͬ���칹��Ľṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��6��A����һ��ͬ���칹�壬�����������̼ԭ����һ��ֱ���ϣ�˵������̼̼��������������ԭ���γ������ǻ����������Ľṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
���������⿼���л����ƶϣ��漰��Ӧ����ʽ����д��ͬ���칹����жϵ�֪ʶ�㣬��Ӧ����ʽ����д��ͬ���칹����ж��Ǹ߿����ȵ㣬Ӧ�������մ�֪ʶ�㣮
 ��
�� =2��3��1�����A����Է�������֪��A�ķ���ʽΪC4H6O2��A�ܷ����ۺϷ�Ӧ����B��˵��A�к���̼̼˫����A��ˮ������C��D��C�ܺ�����������ͭ��Ӧ����D��˵��C�к���ȩ����AΪ������C��D��̼ԭ�Ӹ�����ͬ������C����ȩ��D�����ᣬ��ΪR-CH=CHOH��ϩ�������ȶ����ܿ�ת��ΪR-CH2CHO������A�Ľṹ��ʽΪCH3COOCH=CH2��A�����ۺϷ�Ӧ����B������B�Ľṹ��ʽΪ��
=2��3��1�����A����Է�������֪��A�ķ���ʽΪC4H6O2��A�ܷ����ۺϷ�Ӧ����B��˵��A�к���̼̼˫����A��ˮ������C��D��C�ܺ�����������ͭ��Ӧ����D��˵��C�к���ȩ����AΪ������C��D��̼ԭ�Ӹ�����ͬ������C����ȩ��D�����ᣬ��ΪR-CH=CHOH��ϩ�������ȶ����ܿ�ת��ΪR-CH2CHO������A�Ľṹ��ʽΪCH3COOCH=CH2��A�����ۺϷ�Ӧ����B������B�Ľṹ��ʽΪ�� ��Bˮ�����������E����ϩ����E�Ľṹ��ʽΪ��
��Bˮ�����������E����ϩ����E�Ľṹ��ʽΪ�� ��
������⣺������A��Է�������Ϊ86��̼����������Ϊ55.8%����Ϊ7.0%������Ϊ����A��C��H��Oԭ�ӵ�ԭ�Ӹ���֮��=
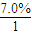 ��
��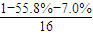 =2��3��1�����A����Է�������֪��A�ķ���ʽΪC4H6O2��A�ܷ����ۺϷ�Ӧ����B��˵��A�к���̼̼˫����A��ˮ������C��D��C�ܺ�����������ͭ��Ӧ����D��˵��C�к���ȩ����AΪ������C��D��̼ԭ�Ӹ�����ͬ������C����ȩ��D�����ᣬ��ΪR-CH=CHOH��ϩ�������ȶ����ܿ�ת��ΪR-CH2CHO������A�Ľṹ��ʽΪCH3COOCH=CH2��A�����ۺϷ�Ӧ����B������B�Ľṹ��ʽΪ��
=2��3��1�����A����Է�������֪��A�ķ���ʽΪC4H6O2��A�ܷ����ۺϷ�Ӧ����B��˵��A�к���̼̼˫����A��ˮ������C��D��C�ܺ�����������ͭ��Ӧ����D��˵��C�к���ȩ����AΪ������C��D��̼ԭ�Ӹ�����ͬ������C����ȩ��D�����ᣬ��ΪR-CH=CHOH��ϩ�������ȶ����ܿ�ת��ΪR-CH2CHO������A�Ľṹ��ʽΪCH3COOCH=CH2��A�����ۺϷ�Ӧ����B������B�Ľṹ��ʽΪ�� ��Bˮ�����������E����ϩ����E�Ľṹ��ʽΪ��
��Bˮ�����������E����ϩ����E�Ľṹ��ʽΪ��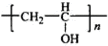 ��
����1��ͨ�����Ϸ���֪��A�ķ���ʽC4H6O2���ʴ�Ϊ��C4H6O2��
��2��C����ȩ����ȩ��������ͭ��Ӧ�������ᡢ������ͭ��ˮ����Ӧ����ʽΪ��CH3CHO+2Cu��OH��2
 CH3COOH+Cu2O+2H2O��
CH3COOH+Cu2O+2H2O���ʴ�Ϊ��CH3CHO+2Cu��OH��2
 CH3COOH+Cu2O+2H2O��
CH3COOH+Cu2O+2H2O����3��ͨ�����Ϸ���֪��A�Ľṹ��ʽΪ��CH3COOCH=CH2���ʴ�Ϊ��CH3COOCH=CH2��
��4��B�Ľṹ��ʽΪ��
 ��Bˮ�����������E����ϩ������Ӧ����ʽΪ��
��Bˮ�����������E����ϩ������Ӧ����ʽΪ�� ��
���ʴ�Ϊ��
 ��
����5��A�ж���ͬ���칹�壬д������ͬʱ���㣨i���ܷ���ˮ�ⷴӦ��˵��������������ii����ʹ������Ȼ�̼��Һ��ɫ��˵������̼̼˫���������������������A��ͬ���칹��Ľṹ��ʽΪ
 ��
���ʴ�Ϊ��
 ��
����6��A����һ��ͬ���칹�壬�����������̼ԭ����һ��ֱ���ϣ�˵������̼̼��������������ԭ���γ������ǻ����������Ľṹ��ʽΪ
 ��
���ʴ�Ϊ��
 ��
�����������⿼���л����ƶϣ��漰��Ӧ����ʽ����д��ͬ���칹����жϵ�֪ʶ�㣬��Ӧ����ʽ����д��ͬ���칹����ж��Ǹ߿����ȵ㣬Ӧ�������մ�֪ʶ�㣮

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
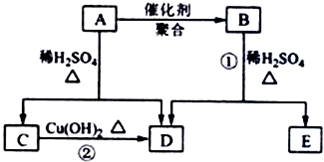 ������A��Է�������Ϊ86��̼����������Ϊ55.8%����Ϊ7.0%������Ϊ����A����ط�Ӧ��ͼ��ʾ��
������A��Է�������Ϊ86��̼����������Ϊ55.8%����Ϊ7.0%������Ϊ����A����ط�Ӧ��ͼ��ʾ��