��Ŀ����
��2009?���գ�A������������һ�ֽྻ������������Դ��������������Ҫ�ɷ�ΪCO��CO2��H2�ȣ���H2��ϣ����ϳɼ״��������������õķ���֮һ��
��1��������Ӧ�Ĵ�������Cu��Zn��Al��Ԫ�أ�д����̬Znԭ�ӵĺ�������Ų�ʽ
��2�����ݵȵ���ԭ����д��CO���ӽṹʽ
��3���״��������ɵõ���ȩ����ȩ������Cu��OH��2�ļ�����Һ��Ӧ����Cu2O������
�ټ״��ķе�ȼ�ȩ�ĸߣ�����Ҫԭ����
�ڼ�ȩ���ӵĿռ乹����
����1��Cu2O�����У��ṹ��ͼ��ʾ������������Cuԭ����ĿΪ
��1��������Ӧ�Ĵ�������Cu��Zn��Al��Ԫ�أ�д����̬Znԭ�ӵĺ�������Ų�ʽ
1s22s22p63s23p63d104s2��[Ar]3d104s2
1s22s22p63s23p63d104s2��[Ar]3d104s2
����2�����ݵȵ���ԭ����д��CO���ӽṹʽ
C��O
C��O
��
��3���״��������ɵõ���ȩ����ȩ������Cu��OH��2�ļ�����Һ��Ӧ����Cu2O������
�ټ״��ķе�ȼ�ȩ�ĸߣ�����Ҫԭ����
�״�����֮���γ����
�״�����֮���γ����
����ȩ������̼ԭ�ӹ�����ӻ�����Ϊsp2�ӻ�
sp2�ӻ�
���ڼ�ȩ���ӵĿռ乹����
ƽ��������
ƽ��������
��1mol��ȩ�����ЦҼ�����ĿΪ3NA
3NA
������1��Cu2O�����У��ṹ��ͼ��ʾ������������Cuԭ����ĿΪ
4
4
����������1������п��ԭ����������ԭ������д��̬Znԭ�ӵĺ�������Ų�ʽ��
��2�����ݵȵ���ԭ������дCO�Ľṹʽ��
��3��������������������ʵķе㣬���ü�ȩ�еijɼ�������̼ԭ�ӵ��ӻ����ͣ�
�������ӻ������������ռ�ṹ���������жϦҼ��Ĺ����������Ҽ���Ŀ��
�����þ����ṹͼ������ͭԭ�ӵ�λ������������Ŀ��
��2�����ݵȵ���ԭ������дCO�Ľṹʽ��
��3��������������������ʵķе㣬���ü�ȩ�еijɼ�������̼ԭ�ӵ��ӻ����ͣ�
�������ӻ������������ռ�ṹ���������жϦҼ��Ĺ����������Ҽ���Ŀ��
�����þ����ṹͼ������ͭԭ�ӵ�λ������������Ŀ��
����⣺��1����Zn��ԭ������Ϊ30��3d����������3d���д��4s�����ǰ�棬������Ų�Ϊ1s22s22p63s23p63d104s2��[Ar]3d104s2���ʴ�Ϊ��1s22s22p63s23p63d104s2��[Ar]3d104s2��
��2�����ݵȵ���ԭ������֪CO��N2Ϊ�ȵ����壬N2���ӵĽṹʽΪN��N����Ϊ�ȵ�������ӵĽṹ���ƣ���CO�ĽṹʽΪC��O���ʴ�Ϊ��C��O��
��3���ټ״�����֮���γ��˷��Ӽ��������ȩ���Ӽ�ֻ�Ƿ��Ӽ�����������û���γ�������ʼ״��ķе�ߣ���ȩ�����к���̼��˫��������3���Ҽ�����̼ԭ�ӹ�����ӻ�����Ϊsp2�ӻ����ʴ�Ϊ���״�����֮���γ������sp2�ӻ���
�����ȩ��̼ԭ�Ӳ�ȡsp2�ӻ�������ӵĿռ乹��Ϊƽ�������Σ�1mol��ȩ�����к���2mol̼��Ҽ���1mol̼���Ҽ����ʺ��ЦҼ�����ĿΪ3NA���ʴ�Ϊ��ƽ�������Σ�3NA��
�����ݾ���ʾ��ͼ���Կ���Cuԭ�Ӵ��ھ����ڲ���Ϊ���������У���������Cuԭ����ĿΪ4���ʴ�Ϊ��4��
��2�����ݵȵ���ԭ������֪CO��N2Ϊ�ȵ����壬N2���ӵĽṹʽΪN��N����Ϊ�ȵ�������ӵĽṹ���ƣ���CO�ĽṹʽΪC��O���ʴ�Ϊ��C��O��
��3���ټ״�����֮���γ��˷��Ӽ��������ȩ���Ӽ�ֻ�Ƿ��Ӽ�����������û���γ�������ʼ״��ķе�ߣ���ȩ�����к���̼��˫��������3���Ҽ�����̼ԭ�ӹ�����ӻ�����Ϊsp2�ӻ����ʴ�Ϊ���״�����֮���γ������sp2�ӻ���
�����ȩ��̼ԭ�Ӳ�ȡsp2�ӻ�������ӵĿռ乹��Ϊƽ�������Σ�1mol��ȩ�����к���2mol̼��Ҽ���1mol̼���Ҽ����ʺ��ЦҼ�����ĿΪ3NA���ʴ�Ϊ��ƽ�������Σ�3NA��
�����ݾ���ʾ��ͼ���Կ���Cuԭ�Ӵ��ھ����ڲ���Ϊ���������У���������Cuԭ����ĿΪ4���ʴ�Ϊ��4��
������������Ҫ�����������Ų�ʽ���ȵ�����ԭ�������Ӽ����������ӻ���������ۼ����͡����ӵ�ƽ�湹�ͣ�ע���˶����ʽṹ�г���������ۺϣ�ѧ���״����ڵ����Ų���3d��4s����д�ϼ��ӻ����͵��ж��ϣ�

��ϰ��ϵ�д�
�����Ŀ
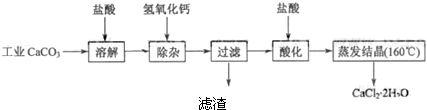
 ��2009?���գ��������и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת�����ǣ�������
��2009?���գ��������и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת�����ǣ�������