��Ŀ����
A��F��ΪԪ�����ڱ���ǰ������Ԫ�أ��������Ϣ���±���
��ش��������⣺
��1��D�ļ۵��ӵĵ����Ų�ʽ�� ��Fԭ�ӵ�ԭ�ӽṹʾ��ͼΪ ��
��2��A��B�ĵ�һ�����ܵĴ�С˳��Ϊ ��
��3��AB3-��Aԭ�ӵ��ӻ��������Ϊ ����A2B��Ϊ�ȵ�����ķ��ӵķ���ʽΪ ����дһ�����ɣ���
��4��D����ľ�����ͼ��ʾΪ�����������ܼ��ѻ����ھ����Ķ�������ľ�����һ��Dԭ�ӣ�����D�ľ�����Dԭ�ӵ���λ��Ϊ ��

��5����֪17g A�ļ��⻯�������������̬ˮʱ�ų�Q kJ����������д��A�ļ��⻯����������Ȼ�ѧ��Ӧ����ʽ ��
��6��C2B2�ĵ���ʽΪ ��������E�Ķ��Ȼ�����Һ��Ӧ������Ӧ��C2B2��E�Ķ��Ȼ�������ʵ���֮��Ϊ1��2����÷�Ӧ�Ļ�ѧ����ʽΪ ��
| Ԫ�� | �����Ϣ |
| A | A�Ļ�̬ԭ�����������Ų�ʽΪ2S22P3 |
| B | B�ǵؿ��к�����ߵ�Ԫ�� |
| C | C+��B�ļ����ӵĵ��Ӳ�ṹ��ͬ |
| D | D��һ�ֺ��ص�������Ϊ64��������Ϊ35 |
| E��F | E��F��ͬ������ͬ�壬��ԭ������F��E��2 |
��1��D�ļ۵��ӵĵ����Ų�ʽ��
��2��A��B�ĵ�һ�����ܵĴ�С˳��Ϊ
��3��AB3-��Aԭ�ӵ��ӻ��������Ϊ
��4��D����ľ�����ͼ��ʾΪ�����������ܼ��ѻ����ھ����Ķ�������ľ�����һ��Dԭ�ӣ�����D�ľ�����Dԭ�ӵ���λ��Ϊ

��5����֪17g A�ļ��⻯�������������̬ˮʱ�ų�Q kJ����������д��A�ļ��⻯����������Ȼ�ѧ��Ӧ����ʽ
��6��C2B2�ĵ���ʽΪ
������ǰ������Ԫ���У�A�Ļ�̬ԭ�����������Ų�ʽΪ2S22P3����AΪN��B�ǵؿ��к�����ߵ�Ԫ�أ���BΪO��C+��B�ļ����ӵĵ��Ӳ�ṹ��ͬ����CΪNa��D��һ�ֺ��ص�������Ϊ64��������Ϊ35������������=64-35=29����DΪCu��E��F��ͬ������ͬ�壬Ϊ����Ԫ�أ���ԭ������F��E��2����EΪFe��FΪNi���ݴ˽��
����⣺ǰ������Ԫ���У�A�Ļ�̬ԭ�����������Ų�ʽΪ2S22P3����AΪN��B�ǵؿ��к�����ߵ�Ԫ�أ���BΪO��C+��B�ļ����ӵĵ��Ӳ�ṹ��ͬ����CΪNa��D��һ�ֺ��ص�������Ϊ64��������Ϊ35������������=64-35=29����DΪCu��E��F��ͬ������ͬ�壬Ϊ����Ԫ�أ���ԭ������F��E��2����EΪFe��FΪNi��
��1��DΪCu��ԭ�Ӻ��������Ϊ29�����������ԭ��������Χ�����Ų�Ϊ��3d104S1��FΪNi��ԭ�Ӻ��������Ϊ28�����ݺ�������Ų����ɣ���ԭ�ӽṹʾ��ͼΪ��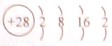 ��
��
�ʴ�Ϊ��3d104S1��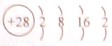 ��
��
��2��N��Oͬ���ڣ�Nԭ��2p�ܼ�����3�����ӣ����ڰ����ȶ�״̬�������ϵͣ���һ�����ܱ�OԪ�ظߣ��ʵ�һ������N��O��
�ʴ�Ϊ��N��O��
��3��NO3-��Nԭ�Ӽ۲���Ӷ���=3+
=3����NԪ�ز�ȡsp2�ӻ�����2��Nԭ����1��C��Oԭ���滻�ɵ���N2O��Ϊ�ȵ�����ķ���ΪCO2��
�ʴ�Ϊ��sp2��CO2��
��4����Cu����ľ����ṹΪ�����������ܼ��ѻ����Զ���Cuԭ��Ϊ�о�������֮�����Cuԭ��λ�������ϣ�1������Cuԭ��Ϊ12���湲�ã���Cuԭ�ӵ���λ��Ϊ12��
�ʴ�Ϊ��12��
��5�����Ĵ�����Ϊ��4NH3+5O2�T4NO+6H2O��g������֪17gNH3������������̬ˮʱ�ų�Q kJ����������4mol������Ӧ�ų�������=4QkJ����NH3���������Ȼ�ѧ��Ӧ����ʽΪ��4NH3��g��+5O2�T4NO��g��+6H2O��g����H=-4QkJ/mol��
�ʴ�Ϊ��4NH3��g��+5O2�T4NO��g��+6H2O��g����H=-4QkJ/mol��
��6��Na2O2������������������ӹ��ɣ���������������ԭ��֮���γ�1�Թ��õ��Ӷ���Oԭ������8���ӽṹ���ʹ������Ƶ���ʽΪ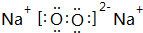 ��������FeCl2��Һ��Ӧ������Ӧ��Na2O2��FeCl2�����ʵ���֮��Ϊ1��2���������ƾ���ǿ�����ԣ���������������Ϊ�����ӣ����ݵ���ת���غ��֪����������ǡ�ñ���ȫ��������Ӧ����NaCl�����������غ��֪����FeCl3�����Ԫ���غ��֪����������������ƽ��÷�Ӧ�Ļ�ѧ����ʽΪ3Na2O2+6FeCl2+6H2O�T6NaCl+4Fe��OH��3��+2FeCl3��
��������FeCl2��Һ��Ӧ������Ӧ��Na2O2��FeCl2�����ʵ���֮��Ϊ1��2���������ƾ���ǿ�����ԣ���������������Ϊ�����ӣ����ݵ���ת���غ��֪����������ǡ�ñ���ȫ��������Ӧ����NaCl�����������غ��֪����FeCl3�����Ԫ���غ��֪����������������ƽ��÷�Ӧ�Ļ�ѧ����ʽΪ3Na2O2+6FeCl2+6H2O�T6NaCl+4Fe��OH��3��+2FeCl3��
�ʴ�Ϊ�� ��3Na2O2+6FeCl2+6H2O�T6NaCl+4Fe��OH��3��+2FeCl3��
��3Na2O2+6FeCl2+6H2O�T6NaCl+4Fe��OH��3��+2FeCl3��
��1��DΪCu��ԭ�Ӻ��������Ϊ29�����������ԭ��������Χ�����Ų�Ϊ��3d104S1��FΪNi��ԭ�Ӻ��������Ϊ28�����ݺ�������Ų����ɣ���ԭ�ӽṹʾ��ͼΪ��
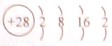 ��
���ʴ�Ϊ��3d104S1��
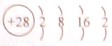 ��
����2��N��Oͬ���ڣ�Nԭ��2p�ܼ�����3�����ӣ����ڰ����ȶ�״̬�������ϵͣ���һ�����ܱ�OԪ�ظߣ��ʵ�һ������N��O��
�ʴ�Ϊ��N��O��
��3��NO3-��Nԭ�Ӽ۲���Ӷ���=3+
| 5+1-2��3 |
| 2 |
�ʴ�Ϊ��sp2��CO2��
��4����Cu����ľ����ṹΪ�����������ܼ��ѻ����Զ���Cuԭ��Ϊ�о�������֮�����Cuԭ��λ�������ϣ�1������Cuԭ��Ϊ12���湲�ã���Cuԭ�ӵ���λ��Ϊ12��
�ʴ�Ϊ��12��
��5�����Ĵ�����Ϊ��4NH3+5O2�T4NO+6H2O��g������֪17gNH3������������̬ˮʱ�ų�Q kJ����������4mol������Ӧ�ų�������=4QkJ����NH3���������Ȼ�ѧ��Ӧ����ʽΪ��4NH3��g��+5O2�T4NO��g��+6H2O��g����H=-4QkJ/mol��
�ʴ�Ϊ��4NH3��g��+5O2�T4NO��g��+6H2O��g����H=-4QkJ/mol��
��6��Na2O2������������������ӹ��ɣ���������������ԭ��֮���γ�1�Թ��õ��Ӷ���Oԭ������8���ӽṹ���ʹ������Ƶ���ʽΪ
 ��������FeCl2��Һ��Ӧ������Ӧ��Na2O2��FeCl2�����ʵ���֮��Ϊ1��2���������ƾ���ǿ�����ԣ���������������Ϊ�����ӣ����ݵ���ת���غ��֪����������ǡ�ñ���ȫ��������Ӧ����NaCl�����������غ��֪����FeCl3�����Ԫ���غ��֪����������������ƽ��÷�Ӧ�Ļ�ѧ����ʽΪ3Na2O2+6FeCl2+6H2O�T6NaCl+4Fe��OH��3��+2FeCl3��
��������FeCl2��Һ��Ӧ������Ӧ��Na2O2��FeCl2�����ʵ���֮��Ϊ1��2���������ƾ���ǿ�����ԣ���������������Ϊ�����ӣ����ݵ���ת���غ��֪����������ǡ�ñ���ȫ��������Ӧ����NaCl�����������غ��֪����FeCl3�����Ԫ���غ��֪����������������ƽ��÷�Ӧ�Ļ�ѧ����ʽΪ3Na2O2+6FeCl2+6H2O�T6NaCl+4Fe��OH��3��+2FeCl3���ʴ�Ϊ��
 ��3Na2O2+6FeCl2+6H2O�T6NaCl+4Fe��OH��3��+2FeCl3��
��3Na2O2+6FeCl2+6H2O�T6NaCl+4Fe��OH��3��+2FeCl3������������ṹ����λ�ù�ϵ�ۺ�Ӧ�ã��漰��������Ų����ӻ�������ȵ����塢�Ȼ�ѧ����ʽ�������ṹ��������ԭ��Ӧ������ʽ�ȣ���Ŀ�ۺ��Խϴ�Ϊ����֪ʶ�Ŀ��飬��6���з���ʽ����дΪ�״��㡢�ѵ㣬�����غ��жϲ��P�����ʵĻ�ѧ���������ѵ��еȣ�

��ϰ��ϵ�д�
 �㽭��У��ʦ���ϵ�д�
�㽭��У��ʦ���ϵ�д�
�����Ŀ
