��Ŀ����
��֪X��Y��ZΪ�����ɶ�����Ԫ�ع��ɵ����ӣ�ÿĦ���Ӷ���10mol���ӣ���ṹ�ص����£�
| X | Y | Z | |
| ԭ�Ӻ��� | ���� | �ĺ� | ˫�� |
| ���ӵĵ���� | 1����λ����� | 0 | 1����λ����� |
����A��B��C��D��E��F��G��H��I��J��K�����ʣ�����֮������Ӧת����ϵ����ͼ��ʾ��ͼ��ʾ��
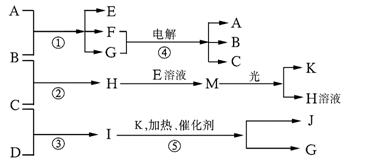
��֪����A��X��Z���ɣ� B��C��D��K���ǵ��ʣ�GΪ��ɫҺ�壬I��ˮ��Һ�ʼ��ԣ���Ӧ�١��ݶ������ڹ�ҵ�����ķ�Ӧ����ش��������⣺?
��1�� A�ĵ���ʽΪ ��D�ĽṹʽΪ ��
��2������B�����Ԫ����Ԫ�����ڱ��е�λ���� ��
��3����Ӧ�ٵĻ�ѧ����ʽΪ ��?
��Ӧ�����ӷ���ʽΪ ��?
��4��H��I��Ӧ���������ˮ��Һ�����ԣ���ԭ���� ����һ�����ӷ�Ӧ����ʽ��ʾ��
��5����֪ÿ����1mol I�ų�46��0kJ����������Ӧ�۵��Ȼ�ѧ����ʽΪ ��
![]() (1)A�� (2��) D��N �� N ��2�֣�
(1)A�� (2��) D��N �� N ��2�֣�
(2) �������ڵڢ�A�壨2�֣�
��3��Cl2 + 2NaOH = NaCl +NaClO + H2O��2�֣�
![]() 2Cl��+2H2O 2OH�� + Cl2�� + H2�� ��2�֣�
2Cl��+2H2O 2OH�� + Cl2�� + H2�� ��2�֣�
(4)NH4++H2O ![]() NH3��H2O+H+��2�֣�
NH3��H2O+H+��2�֣�
��5��N2��g��+3H2(g)= 2NH3(g) ��H=��92��0kJ��3��,ֻҪ�𰸺������ɵ÷�
����:

��֪X��Y��ZΪ����ԭ������������Ԫ�أ�����������Ӧˮ������������ǿ���ǣ�
HXO4��H2YO4��H3ZO4��������˵����ȷ����
| A����̬�⻯����ȶ��ԣ�HX��H2Y��ZH3? | B���ǽ��������ԣ�Y��X��Z? |
| C��ԭ�Ӱ뾶��X��Y��Z? | D��ԭ��������Z��Y��X |