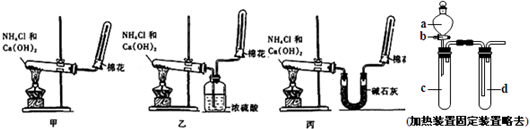
��Ŀ����
����SO2���ŷš���������SO2��Ϊ�����Ե��о����⣮�ҹ��о���Ա���Ƶ����õ�Ʒλ���̿���Ҫ�ɷ���MnO2�����շ������±��ղ�����SO2���Ʊ������̵������������£�

����Һ��pH��2�����еĽ���������Ҫ��Mn2+��������������Fe2+��Al3+��Ca2+��Pb2+�������������ӣ�
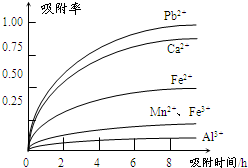
�йؽ������ӵİ뾶�Լ��γ������������ʱ��pH���±��������������������������ӵ�Ч������ͼ��
| ���� | ���Ӱ뾶��pm�� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2+ | 74 | 7.6 | 9.7 |
| Fe3+ | 64 | 2.7 | 3.7 |
| Al3+ | 50 | 3.8 | 4.7 |
| Mn2+ | 80 | 8.3 | 9.8 |
| Pb2+ | 121 | 8.0 | 8.8 |
| Ca2+ | 99 | - | - |
 ��֪PbO2�������Դ���MnO2����ش��������⣺
��֪PbO2�������Դ���MnO2����ش��������⣺��1��д��������������Ҫ��Ӧ�Ļ�ѧ����ʽ______��������������Ҫ��Ӧ�����ӷ���ʽ______��
��2�����������Һ���м���ʯ�ҽ������ڵ���pH��pHӦ������______��
��3�����������������ڳ�ȥ���ʽ������ӣ���������������������Ч����������______����д��ţ���
a����Һ��pH������ b���������ӵĵ�ɡ����� c���������ӵİ뾶������ d������ʱ��
��4������a����______�ȹ��̣�
�⣺��1����Ʒλ���̿���Ҫ�ɷ���MnO2��ͨ��SO2����Һ��pH��2�����еĽ���������Ҫ��Mn2+����MnO2��SO2����������ԭ��Ӧ����Ӧ�Ļ�ѧ����ʽΪSO2+MnO2=MnSO4������������ֻ��Fe2+���л�ԭ�ԣ����Ա�MnO2������������������Fe3+����Ӧ�����ӷ���ʽΪ2Fe2++MnO2+4H+=2Fe3++Mn2++2H2O��
�ʴ�Ϊ��SO2+MnO2=MnSO4��2Fe2++MnO2+4H+=2Fe3++Mn2++2H2O��
��2�������к���Fe2+��Al3+��Ca2+��Pb2+���������ӣ��ɳ�����pH��Χ֪��Fe2+�ij�����Mn2+���ӵij��������pH�ӽ�����Fe3+������Զ���ʿ��Խ�Fe2+������Fe3+�����ӣ��������ʵ�ͼ���Կ�����Ca2+��Pb2+�������ʽϸߣ���ֻҪ����pHֵ��4.7��8.3�䣬����4.7���Խ�Fe3+��Al3+��ȥ��С��8.3�Ƿ�ֹMn2+Ҳ������
�ʴ�Ϊ��4.7��8.3��
��3��ͼ�����Ӵ������£��뾶�м�С���ƣ���Ӧ�������ʼ�С������ʱ��ĵ������������ӵ������ʾ�������Fe3+��Al3+����������������������ʵͣ�
�ʴ�Ϊ��b��c��d��
��4������Һ�л�����ʣ�Ӧ����������Ũ�����ᾧ�ķ������ʴ�Ϊ��������Ũ�����ᾧ��
��������1��������ͼ�����������������̿�MnO2����SO2�ķ�Ӧ������������ֻ��Fe2+���л�ԭ�ԣ����Ա�MnO2������������������Fe3+��
��2���������ʵ�ͼ���Կ�����Ca2+��Pb2+�������ʽϸߣ���ֻҪ����pHֵ��4.7��8.3�䣬����4.7���Խ�Fe3+��Al3+��ȥ��С��8.3�Ƿ�ֹMn2+Ҳ������
��3����ϰ뾶��������ͼ֪��ͼ�����Ӵ������£��뾶�м�С���ƣ���Ӧ�������ʼ�С������ʱ��ĵ������������ӵ������ʾ�������Fe3+��Al3+����������������������ʵͣ�
��4��������ȡ��MnSO4?H2O���нᾧˮ���ʲ�������Ũ���ᾧ�ķ�����
�������������Ʊ������̵���������Ϊ֪ʶ���壬���黯ѧ��Ӧ����д���������е����⣬��Ŀ�Ѷ��еȣ�����ע��������ݴ���������ͼ�����������
�ʴ�Ϊ��SO2+MnO2=MnSO4��2Fe2++MnO2+4H+=2Fe3++Mn2++2H2O��
��2�������к���Fe2+��Al3+��Ca2+��Pb2+���������ӣ��ɳ�����pH��Χ֪��Fe2+�ij�����Mn2+���ӵij��������pH�ӽ�����Fe3+������Զ���ʿ��Խ�Fe2+������Fe3+�����ӣ��������ʵ�ͼ���Կ�����Ca2+��Pb2+�������ʽϸߣ���ֻҪ����pHֵ��4.7��8.3�䣬����4.7���Խ�Fe3+��Al3+��ȥ��С��8.3�Ƿ�ֹMn2+Ҳ������
�ʴ�Ϊ��4.7��8.3��
��3��ͼ�����Ӵ������£��뾶�м�С���ƣ���Ӧ�������ʼ�С������ʱ��ĵ������������ӵ������ʾ�������Fe3+��Al3+����������������������ʵͣ�
�ʴ�Ϊ��b��c��d��
��4������Һ�л�����ʣ�Ӧ����������Ũ�����ᾧ�ķ������ʴ�Ϊ��������Ũ�����ᾧ��
��������1��������ͼ�����������������̿�MnO2����SO2�ķ�Ӧ������������ֻ��Fe2+���л�ԭ�ԣ����Ա�MnO2������������������Fe3+��
��2���������ʵ�ͼ���Կ�����Ca2+��Pb2+�������ʽϸߣ���ֻҪ����pHֵ��4.7��8.3�䣬����4.7���Խ�Fe3+��Al3+��ȥ��С��8.3�Ƿ�ֹMn2+Ҳ������
��3����ϰ뾶��������ͼ֪��ͼ�����Ӵ������£��뾶�м�С���ƣ���Ӧ�������ʼ�С������ʱ��ĵ������������ӵ������ʾ�������Fe3+��Al3+����������������������ʵͣ�
��4��������ȡ��MnSO4?H2O���нᾧˮ���ʲ�������Ũ���ᾧ�ķ�����
�������������Ʊ������̵���������Ϊ֪ʶ���壬���黯ѧ��Ӧ����д���������е����⣬��Ŀ�Ѷ��еȣ�����ע��������ݴ���������ͼ�����������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
����ʵ����������Ӧ�����ӷ���ʽ����ȷ����
| ʵ�� | ���� | ���ӷ���ʽ | |
| A |  K2SO4 K2SO4Kl������Һ | �ڿ��������һ��ʱ�����Һ����ɫ | 4H++4I-+O2�T2I2+2H2O |
| B |  ϡ���� ϡ����ϡ̼������Һ | ��ʼʱ�����ݣ����������� | CO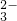 +H+�THCO +H+�THCO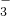 HCO 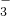 +H+�TH2O+CO2�� +H+�TH2O+CO2�� |
| C |  NaOH��Һ NaOH��ҺCl2ˮ | ��Һ�ɻ���ɫ��Ϊ��ɫ | Cl2+2OH-�TCl-+ClO-+H2O |
| D |  H2SO4���з�̪�� H2SO4���з�̪��Ba��OH��2��Һ | �а�ɫ�������ɣ���Һ�ɺ�ɫ��Ϊ��ɫ | Ba2++OH-+H++SO42-�TBaSO4��+H2O |
- A.A
- B.B
- C.C
- D.D