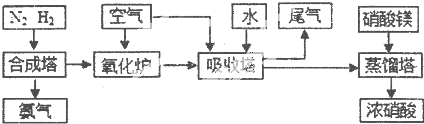
��Ŀ����
T��ʱ����1L�ܱ�������A������B���巴Ӧ����C���塣��Ӧ������A��B��CŨ�ȱ仯��ͼI��ʾ�������������������䣬�¶ȷֱ�ΪTl��T2ʱ��B������ٷֺ�����ʱ��Ĺ�ϵ��ͼ����ʾ�������н�����ȷ���� ( )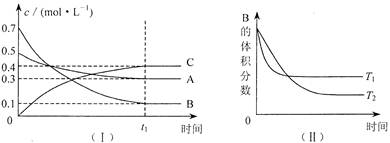
A���ڴ�ƽ����������������䣬����ѹǿ��ƽ��������Ӧ�����ƶ�
B���ڴ�ƽ�����ѹǿ���䣬ͨ��ϡ�����壬ƽ��������Ӧ�����ƶ�
C�����������������䣬����Ӧ��ʼʱA��B��C��Ũ�ȷֱ�Ϊ0.4mo1��L-1��0.5 mo1��L-1��0.2 mo1��L��1����ﵽƽ���C��Ũ��С��0.4 mo1��L-1
D�����������������䣬�����¶ȣ������淴Ӧ���ʾ�������A��ת��������
A
������ȷ�𰸣�A
A����ͼI��A��B��C�ļ�����֮��Ϊ0.2��0.6��0.4=1��3��2������ʽΪA��3B 2C,�����������С�ķ�Ӧ�����������������䣬����ѹǿ��ƽ��������Ӧ�����ƶ���
2C,�����������С�ķ�Ӧ�����������������䣬����ѹǿ��ƽ��������Ӧ�����ƶ���
B������ȷ���ڴ�ƽ�����ѹǿ���䣬ͨ��ϡ�����壬������ͣ�ƽ�����淴Ӧ�����ƶ�
C������ȷ�����������������䣬����Ӧ��ʼʱA��B��C��Ũ�ȷֱ�Ϊ0.4mo1��L-1��0.5 mo1��L-1��0.2 mo1��L��1����0.2 mo1��L��1ȫ��ת����A��B���൱��������0.��mo1��L-1A��0.8mo1��L-1B��B��ԭ����ƽ�������ƶ�����ﵽƽ���C��Ũ�ȴ���0.4 mo1��L-1
D������ȷ����ͼ���������������䣬�����¶ȣ�B�������������ƽ�������ƶ��������淴Ӧ���ʾ�����A��ת���ʼ�С��


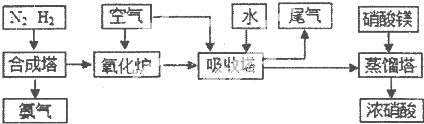
��1���ٹ�ҵ����ʱ����ȡ������һ����ӦΪ��CO+H2O��g��?CO2+H2��850��ʱ����1L�ܱ������г���0.3mol CO��0.2molH2O��g������Ӧ4min����ƽ�⣬��ϵ��c��H2��=0.12mol?L-1��CO��ƽ��Ũ��Ϊ ת����Ϊ ���¶��´˷�Ӧ��ƽ�ⳣ��K= �������������
����850��ʱ���Ա��е����ʵ���Ͷ����ݷ�Ӧ���У��������淴Ӧ������е��� ��ѡ��A��B��C��D��E��
| A | B | C | D | E | |
| n��CO2�� | 3 | l | 1 | l | |
| n��H2�� | 2 | l | 1 | 2 | |
| n��CO�� | 1 | 2 | 3 | 0.5 | 3 |
| n��H2O�� | 5 | 2 | 3 | 2 | l |
| T/°C | T1 | 300 | T2 |
| K | 1.00×107 | 2.45×105 | 1.88×103 |
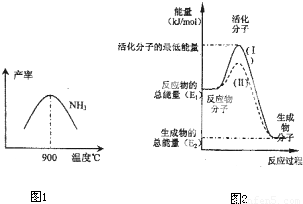
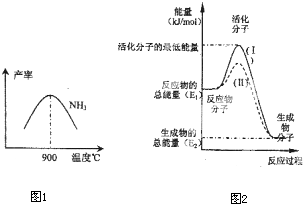
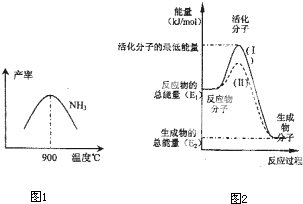
��4���ڻ�ѧ��Ӧ��ֻ�м�����������ƽ�������ߵö�ķ�Ӧ����ӷ�����ײʱ�ſ��ܷ�����ѧ��Ӧ����Щ���ӱ���Ϊ����ӣ�ʹ��ͨ���ӱ�ɻ���������ṩ������ȵ������л�ܣ��䵥λͨ����kJ?mol-1��ʾ��������۲�ͼ2���ش����⣮
ͼ����ʾ��Ӧ�� ������ȡ����ȡ�����Ӧ���÷�Ӧ�ġ�H= ���ú�E1��E2E�Ĵ���ʽ��ʾ������֪�Ȼ�ѧ����ʽ��H2��g��+
 O2��g��=H2O��g����H=-241.8kJ?mol-1���÷�Ӧ�Ļ��Ϊ167.2kJ?mol-1�������淴Ӧ�Ļ��Ϊ ��
O2��g��=H2O��g����H=-241.8kJ?mol-1���÷�Ӧ�Ļ��Ϊ167.2kJ?mol-1�������淴Ӧ�Ļ��Ϊ ����5�����᳧��β��ֱ���ŷŽ���Ⱦ������Ŀǰ��ѧ��̽������ȼ�������еļ���Ƚ����������ﻹԭΪ������ˮ����Ӧ����Ϊ��
CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g����H=-574kJ?mol-1
CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g����H=-1160kJ?mol-1
�����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ�� ��
 CH3OH��g��+H2O��g������H3
CH3OH��g��+H2O��g������H3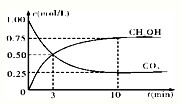
 NH3?H2O+H+
NH3?H2O+H+