��Ŀ����
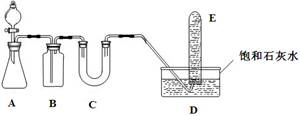
(9��)��ͼ��ijͬѧ��Ƶķ��ȷ�Ӧ�Ĺ۲�װ�á�
��ʵ��˳���ǣ��ٰ�ͼ��ʾ��ʵ��װ�����Ӻá�
����U�ι��ڼ���������īˮ(��Ʒ��)��Һ����T�� �����У�ʹU�ι������ߵ�Һ�洦��ͬһˮƽ�棬�ټн������С������м���Թ���ʢ1 g�����ƣ�������2 mL���ҵ�����ˮ��ͬʱ�������м��ɹ۲졣
�Իش�(1)ʵ���й۲쵽��������
(2)��ʵ���б�����е�һ��ʵ�������
(3)��ʵ���ԭ����
________________________________________________________________________
(4)ʵ���з�Ӧ�Ļ�ѧ��Ӧ����ʽ��
(5)˵��CaO��H2O��������Ca(OH)2������֮��Ĺ�ϵ
(6)����ʵ����CaO����NaCl��ʵ�黹�ܷ�۲쵽��ͬ����____��

��ʵ��˳���ǣ��ٰ�ͼ��ʾ��ʵ��װ�����Ӻá�
����U�ι��ڼ���������īˮ(��Ʒ��)��Һ����T�� �����У�ʹU�ι������ߵ�Һ�洦��ͬһˮƽ�棬�ټн������С������м���Թ���ʢ1 g�����ƣ�������2 mL���ҵ�����ˮ��ͬʱ�������м��ɹ۲졣
�Իش�(1)ʵ���й۲쵽��������
(2)��ʵ���б�����е�һ��ʵ�������
(3)��ʵ���ԭ����
________________________________________________________________________
(4)ʵ���з�Ӧ�Ļ�ѧ��Ӧ����ʽ��
(5)˵��CaO��H2O��������Ca(OH)2������֮��Ĺ�ϵ
(6)����ʵ����CaO����NaCl��ʵ�黹�ܷ�۲쵽��ͬ����____��
��9�֣�(1)U�β�������ĺ�īˮ(��Ʒ��)���ؿ��ڶ�������2�֣�
(2)���װ�������� ��1�֣�
(3)CaO��ˮ��Ӧ�ų�����ʹ���Թ��п������ͣ�ѹǿ���������īˮ(��Ʒ��)��U�ι��е�Һ�治����ƽ ��2�֣�
(4)CaO��H2O===Ca(OH)2 ��1�֣�
(5)CaO��H2O�������ʹ���Ca(OH)2������ ��2�֣� (6)�� ��1�֣�
(2)���װ�������� ��1�֣�
(3)CaO��ˮ��Ӧ�ų�����ʹ���Թ��п������ͣ�ѹǿ���������īˮ(��Ʒ��)��U�ι��е�Һ�治����ƽ ��2�֣�
(4)CaO��H2O===Ca(OH)2 ��1�֣�
(5)CaO��H2O�������ʹ���Ca(OH)2������ ��2�֣� (6)�� ��1�֣�
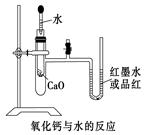
���鷴Ӧ�������Ĺ�ϵ
������ʯ������ˮ�ų��������ȣ����Թ��п������ͣ�ѹǿ��������U�β�������ĺ�īˮ���ؿ��ڶ�����
��ʵ���Ƿ�ɹ��Ĺؼ���Ҫ��֤�����Ե�����
���ڷ��ȷ�Ӧ��������Ӧ�������֮�ʹ����������������
ʳ������ˮ����ЧӦ������
������ʯ������ˮ�ų��������ȣ����Թ��п������ͣ�ѹǿ��������U�β�������ĺ�īˮ���ؿ��ڶ�����
��ʵ���Ƿ�ɹ��Ĺؼ���Ҫ��֤�����Ե�����
���ڷ��ȷ�Ӧ��������Ӧ�������֮�ʹ����������������
ʳ������ˮ����ЧӦ������

��ϰ��ϵ�д�
�����Ŀ