��Ŀ����
�跴Ӧ��Fe(S)+CO2(g)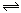 FeO(S)+CO(g)��ƽ�ⳣ��Ϊ
FeO(S)+CO(g)��ƽ�ⳣ��Ϊ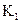 ����Ӧ��Fe(S)+H2O(g)
����Ӧ��Fe(S)+H2O(g)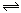 FeO(S)+H2(g)��ƽ�ⳣ��Ϊ
FeO(S)+H2(g)��ƽ�ⳣ��Ϊ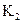 ���ڲ�ͬ�¶��£�
���ڲ�ͬ�¶��£�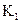 ��
��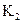 ��ֵ���£�
��ֵ���£�
(1)���ϱ������ƶϣ���Ӧ����_______________ (����������š�)�ȷ�Ӧ��
(2)���з�Ӧ��H2(g)+CO2(g)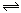 H2O(g)+CO(g)д���÷�Ӧ��ƽ�ⳣ��K3����ѧ����ʽ��K
H2O(g)+CO(g)д���÷�Ӧ��ƽ�ⳣ��K3����ѧ����ʽ��K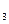 =____________��
=____________��
(3)���ݷ�Ӧ����ڣ����Ƶ���K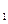 ��K
��K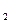 ��K
��K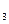 ֮��Ĺ�ϵʽ_______________���ݴ˹�ϵʽ���ϱ����ݣ�Ҳ���ƶϳ���Ӧ���ǡ����ȡ����ǡ����ȡ���Ӧ���ʣ�Ҫʹ��Ӧ����һ�������½�����ƽ��������Ӧ�����ƶ����ɲ�ȡ�Ĵ�ʩ��_____________(��д���)��
֮��Ĺ�ϵʽ_______________���ݴ˹�ϵʽ���ϱ����ݣ�Ҳ���ƶϳ���Ӧ���ǡ����ȡ����ǡ����ȡ���Ӧ���ʣ�Ҫʹ��Ӧ����һ�������½�����ƽ��������Ӧ�����ƶ����ɲ�ȡ�Ĵ�ʩ��_____________(��д���)��
(4)ͼl��2��ʾ�÷�Ӧ����ʱ��t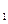 �ﵽƽ�⡢��ʱ��t
�ﵽƽ�⡢��ʱ��t �ֱ���ı�ij�������������仯�������
�ֱ���ı�ij�������������仯�������
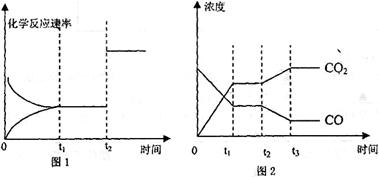
��ͼl��ʱ��t �����ı��������_____________________________
�����ı��������_____________________________
��ͼ2��ʱ��t �����ı��������________________
�����ı��������________________
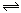 FeO(S)+CO(g)��ƽ�ⳣ��Ϊ
FeO(S)+CO(g)��ƽ�ⳣ��Ϊ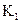 ����Ӧ��Fe(S)+H2O(g)
����Ӧ��Fe(S)+H2O(g)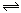 FeO(S)+H2(g)��ƽ�ⳣ��Ϊ
FeO(S)+H2(g)��ƽ�ⳣ��Ϊ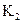 ���ڲ�ͬ�¶��£�
���ڲ�ͬ�¶��£�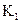 ��
��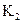 ��ֵ���£�
��ֵ���£�| �¶� | 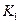 | 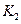 |
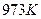 | 1.47 | 2.38 |
1173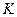 | 2.15 | 1.67 |
(1)���ϱ������ƶϣ���Ӧ����_______________ (����������š�)�ȷ�Ӧ��
(2)���з�Ӧ��H2(g)+CO2(g)
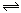 H2O(g)+CO(g)д���÷�Ӧ��ƽ�ⳣ��K3����ѧ����ʽ��K
H2O(g)+CO(g)д���÷�Ӧ��ƽ�ⳣ��K3����ѧ����ʽ��K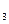 =____________��
=____________��(3)���ݷ�Ӧ����ڣ����Ƶ���K
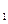 ��K
��K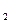 ��K
��K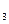 ֮��Ĺ�ϵʽ_______________���ݴ˹�ϵʽ���ϱ����ݣ�Ҳ���ƶϳ���Ӧ���ǡ����ȡ����ǡ����ȡ���Ӧ���ʣ�Ҫʹ��Ӧ����һ�������½�����ƽ��������Ӧ�����ƶ����ɲ�ȡ�Ĵ�ʩ��_____________(��д���)��
֮��Ĺ�ϵʽ_______________���ݴ˹�ϵʽ���ϱ����ݣ�Ҳ���ƶϳ���Ӧ���ǡ����ȡ����ǡ����ȡ���Ӧ���ʣ�Ҫʹ��Ӧ����һ�������½�����ƽ��������Ӧ�����ƶ����ɲ�ȡ�Ĵ�ʩ��_____________(��д���)��| A����С��Ӧ�����ݻ� | B������Ӧ�����ݻ� | C�������¶� |
| D�������¶� E��ʹ�ú��ʵĴ��� F���跨����CO���� |
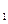 �ﵽƽ�⡢��ʱ��t
�ﵽƽ�⡢��ʱ��t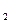 �ֱ���ı�ij�������������仯�������
�ֱ���ı�ij�������������仯�������
��ͼl��ʱ��t
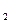 �����ı��������_____________________________
�����ı��������_____________________________��ͼ2��ʱ��t
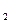 �����ı��������________________
�����ı��������________________��1���� ��2��
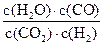 ��3��
��3��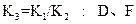
��4���ټ��������������ϵ��ѹǿ �ڽ����¶Ȼ�����ˮ���������������������

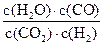 ��3��
��3��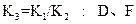
��4���ټ��������������ϵ��ѹǿ �ڽ����¶Ȼ�����ˮ���������������������
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 ������Ϊ�����Ӳ����ڣ�
������Ϊ�����Ӳ����ڣ�
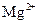 ��������
��������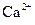
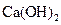 ��
��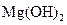 �Ļ����
�Ļ����