��Ŀ����
��12�֣��������ʵ����ܶ࣬���Ӿ��ǹ������ʵĻ�����֮һ���±�����ѧ��ѧ�г����ļ������ӣ�
�ش��������⣺
��1��K+���ӵĽṹʾ��ͼ ��Cl�����ڱ��е�λ���� ��
��2������OH-���ӵ���Һ����Ũ��Ϊ0.1mol/L��Al3+��NH+4�Ļ����Һ�У��������Һ��NH+4������һ�룬���ʱ��Һ�� ������ڡ������ڡ���������Al3+��
��3������A��B�ֱ����ϱ��е�����������ɣ������Ƿֱ�����ˮ�У�A����Һ�����ԣ�B����Һ�Լ�����B����ɫ��Ӧ����ɫ������ɫ�겣���۲죩����A��B����Һ��ϣ����а�ɫ�������ɣ�������ɫ��ζ���������ɡ���A�к��е��������� ��B��Һ�Լ��Ե�ԭ���ǣ������ӷ���ʽ�ͱ�Ҫ������˵���� ��
��4�������£�����NH4��2CO3����ˮ���õ����д���NH4+��CO2-3����Һ������Һ�е���������ˮ�������Һ��NH+4��CO2-3��Ũ�ȱ�Ϊ2��1�����ʱ����Һ��pH ���<���� ��=����>����7��
��5�������������µ�������NH+4�ɱ�����������NO-3��д���÷�Ӧ�����ӷ���ʽ
��
����12�֣���1��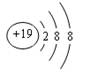 ����1�֣���������������A��2�֣�
����1�֣���������������A��2�֣�
��2�������ڣ�1�֣�
��3��Al3+��1�֣���BΪKHCO3������Һ�е����K����HCO3����HCO3������Һ�д���ˮ��͵�������ƽ�⣺HCO3����H2O H2CO3��OH����HCO3��
H2CO3��OH����HCO3�� CO32����H��������HCO3����ˮ��̶ȴ��ڵ���̶ȣ�������Һ�Լ��ԡ���3�֣�
CO32����H��������HCO3����ˮ��̶ȴ��ڵ���̶ȣ�������Һ�Լ��ԡ���3�֣�
��4���� ( 2�� ) ��5��NH4����2O2 NO3����H2O��2H����2�֣�
NO3����H2O��2H����2�֣�
����

 ��У����ϵ�д�
��У����ϵ�д�