��Ŀ����
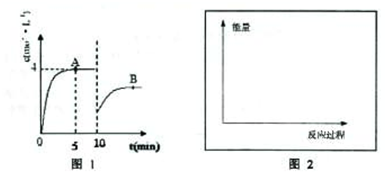
��ͼ1��ʾ���ϳɰ�������ʾʵ�飨�г���������ʡ�ԣ�����Y�ιܵ�һ����Zn����ϡH2SO4��Ӧ��ȡH2����һ����NaNO2�����NH4Cl������Һ��Ӧ��ȡN2��N2��H2��Ϻ�ͨ����ԭ�������ϳ�NH3���ٽ�����������ͨ���̪��Һ�У�����̪��Һ��죬��˵�������˰�����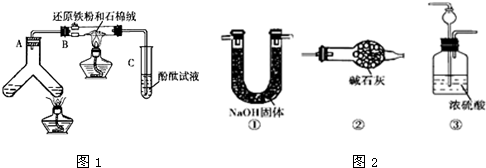
ij����С��ͨ���������ϺͶ��ʵ�飬�õ���������Ϣ��
��Ϣһ��NaNO2����ͱ���NH4Cl��Һ��ϼ��ȵĹ����з������·�Ӧ��
��NaNO2+NH4Cl��NH4NO2+NaCl
��NH4NO2��NH3+HNO2
��2HNO2��N2O3+H2O
��2NH3+N2O3��2N2+3H2O
��Ϣ�����������ϣ���ͬ����ȵ�N2��H2 �����������ͬʵ�������ºϳɰ���ʹ��̪��Һ�������Ҫ��ʱ�����£�
| N2��H2������� | 5��1 | 3��1 | 1��1 | 1��3 | 1��5 |
| ��̪���ɫ����ʱ��/min | 8��9 | 7��8 | 6��7 | 3��4 | 9��10 |
��1��Y�ι������з�����Ӧ�����ӷ���ʽ��
��2����������ʯ�����ϵ�Ŀ����
��3������С���ͬѧ����Ϊ����ʵ���м�ʹ��̪���Ҳ����˵��N2��H2��Ӧ�ϳ���NH3���ó��˽��۵�������
��4��������ʵ������У�Ϊ����۲쵽��̪��Һ����ʵ������Ӧ�ÿ���N2��H2�������Ϊ
��5��ʵ�������ͨ���Թ�C�е�����ɷ���
��������1��Y�ιܵ�һ����Zn����ϡH2SO4��Ӧ��ȡH2����һ����NaNO2�����NH4Cl������Һ��Ӧ��ȡN2��װ��ͼ���Ҳ��Ǽ��ȷ�Ӧ�������Ʊ�����������Ҫ�����Ʊ��������Ʊ�������װ�ã�
��2��N2��H2��Ϻ�ͨ����ԭ�������ϳ�NH3��Ϊ����������һ�����ĽӴ����������������ʯ�����ϣ�
��3������С���ͬѧ����Ϊ����ʵ���м�ʹ��̪��Һ���Ҳ����˵��N2��H2��Ӧ�ϳ���NH3�������ó��˽��۵����ɣ����һ����ʵ����֤������ɣ�
��4��������ʵ������У�Ϊ����۲쵽��̪����ʵ������Ӧ�ÿ���N2��H2�������Ϊ 1��3�Ƚ����ˣ��������������������װ�û�����ʵ�ִ�Ŀ�ģ�
��5������Ŀ��Ϣ���Ʋ�ʵ�������ͨ���Թ�C�е�����ijɷ֣�
��2��N2��H2��Ϻ�ͨ����ԭ�������ϳ�NH3��Ϊ����������һ�����ĽӴ����������������ʯ�����ϣ�
��3������С���ͬѧ����Ϊ����ʵ���м�ʹ��̪��Һ���Ҳ����˵��N2��H2��Ӧ�ϳ���NH3�������ó��˽��۵����ɣ����һ����ʵ����֤������ɣ�
��4��������ʵ������У�Ϊ����۲쵽��̪����ʵ������Ӧ�ÿ���N2��H2�������Ϊ 1��3�Ƚ����ˣ��������������������װ�û�����ʵ�ִ�Ŀ�ģ�
��5������Ŀ��Ϣ���Ʋ�ʵ�������ͨ���Թ�C�е�����ijɷ֣�
����⣺��1��Y�ι������з�����Ӧ�Ļ�ѧ����ʽΪ Zn+H2SO4�TZnSO4+H2����
�ʴ�Ϊ��Zn+H2SO4�TZnSO4+H2����
��2��ʯ���������������������������ԭ���۵ĽӴ������ʹ��Ӧ���еø���֣�
�ʴ�Ϊ�������������������ԭ���۵ĽӴ������ʹ��Ӧ���еø���֣�
��3������С���ͬѧ����Ϊ����ʵ���м�ʹ��̪��Һ���Ҳ����˵��N2��H2��Ӧ�ϳ���NH3���ó��˽��۵������ǣ��ӷֲ���Ӧ��֪������N2�Ĺ����У��п���ֱ�Ӳ��������������һ����ʵ����֤������ɣ���NaNO2����ͱ���NH4Cl��Һ��ϼ��ȣ�����������ֱ��ͨ���̪��Һ������̪��Һ��죬��˵�����ɳ���������˵�����ɲ��������������һ���⣬����ѡ����ͼ�еĢ�װ��������ԭװ���е�A��B֮�䣻
�ܴ�Ϊ���ӷֲ���Ӧ��֪������N2�Ĺ����У��п���ֱ�Ӳ���������
��NaNO2����ͱ���NH4Cl��Һ��ϼ��ȣ�����������ֱ��ͨ���̪��Һ������̪��Һ��죬��˵�����ɳ���������˵�����ɲ������� �ۣ� A��B��
��4��������ʵ������У�Ϊ����۲쵽��̪����ʵ���������ݻ�ѧ��Ӧ������ϵ��N2+3H2?2NH3��Ӧ�ÿ���N2��H2�������Ϊ 1��3�Ƚ����ˣ�����װ�û�����ʵ�ִ�Ŀ��ԭ����������ͨ��B��N2��H2������ȣ�
�ʴ�Ϊ�������Ϊ 1��3�Ƚ����ˣ�������ͨ��B��N2��H2������ȣ�
��5��ʵ������У����ݵ����������ϳɰ��ǿ��淴Ӧ�����ܽ��г��ף�����ͨ���Թ�C�е�����ɷ��� NH3��N2��H2��
�ʴ�Ϊ��NH3��N2��H2��
�ʴ�Ϊ��Zn+H2SO4�TZnSO4+H2����
��2��ʯ���������������������������ԭ���۵ĽӴ������ʹ��Ӧ���еø���֣�
�ʴ�Ϊ�������������������ԭ���۵ĽӴ������ʹ��Ӧ���еø���֣�
��3������С���ͬѧ����Ϊ����ʵ���м�ʹ��̪��Һ���Ҳ����˵��N2��H2��Ӧ�ϳ���NH3���ó��˽��۵������ǣ��ӷֲ���Ӧ��֪������N2�Ĺ����У��п���ֱ�Ӳ��������������һ����ʵ����֤������ɣ���NaNO2����ͱ���NH4Cl��Һ��ϼ��ȣ�����������ֱ��ͨ���̪��Һ������̪��Һ��죬��˵�����ɳ���������˵�����ɲ��������������һ���⣬����ѡ����ͼ�еĢ�װ��������ԭװ���е�A��B֮�䣻
�ܴ�Ϊ���ӷֲ���Ӧ��֪������N2�Ĺ����У��п���ֱ�Ӳ���������
��NaNO2����ͱ���NH4Cl��Һ��ϼ��ȣ�����������ֱ��ͨ���̪��Һ������̪��Һ��죬��˵�����ɳ���������˵�����ɲ������� �ۣ� A��B��
��4��������ʵ������У�Ϊ����۲쵽��̪����ʵ���������ݻ�ѧ��Ӧ������ϵ��N2+3H2?2NH3��Ӧ�ÿ���N2��H2�������Ϊ 1��3�Ƚ����ˣ�����װ�û�����ʵ�ִ�Ŀ��ԭ����������ͨ��B��N2��H2������ȣ�
�ʴ�Ϊ�������Ϊ 1��3�Ƚ����ˣ�������ͨ��B��N2��H2������ȣ�
��5��ʵ������У����ݵ����������ϳɰ��ǿ��淴Ӧ�����ܽ��г��ף�����ͨ���Թ�C�е�����ɷ��� NH3��N2��H2��
�ʴ�Ϊ��NH3��N2��H2��
���������⿼�����������ʵ�ʵ�����Ʊ�������������֤�����������֪ʶ�Ŀ��飬����Ĺؼ��Ǹ��������ṩ����Ϣ������е�֪ʶ���з������������Ի�����չ�����⣬����ѧ����֪ʶǨ������������

��ϰ��ϵ�д�
�����Ŀ