��Ŀ����
��9�֣�1 Lij�����Һ�����ܺ��е� �������±���
�������±���
| ���ܴ������е������� | H+ NH4+ Al3+ K+ |
| ���ܴ������е������� | Cl- Br- I? ClO? AlO2- |
 �����NaOH��Һ�������
�����NaOH��Һ������� ���Ĺ�ϵ����ͼ��ʾ�������Һ��ȷ�����е�������_______________��
���Ĺ�ϵ����ͼ��ʾ�������Һ��ȷ�����е�������_______________������ȷ���Ƿ��е���������__________��Ҫȷ������ڿɲ�������ʵ����________��

��2������⣬����Һ�к��д�����Cl�� ��Br����I��������1 L�û����Һ��ͨ�롪������Cl2����Һ��Cl����Br����I�������ʵ�����ͨ��Cl2�������״������
 ��ϵ���±���ʾ��������ش��������⣺
��ϵ���±���ʾ��������ش��������⣺| Cl2���������״���� | 2.8L | 5.6 L | 11.2 L |
 ��Cl���� ��Cl���� | 1.25mol | 1.5 mol | 2 mol |
 ��Br���� ��Br���� | 1.5 mol | 1.4 mol | 0.9 mol |
 ��I���� ��I���� |  mol mol | 0 | 0 |
��ԭ��Һ��Cl����Br����I�������ʵ���Ũ��֮��Ϊ_____________��
��1��H+��Al3+��NH4+��3�֣� K����1�֣���ɫ��Ӧ��1�֣�
��2����Cl2��2I����I2��2Cl����2�֣� �� 10��15��4��2�֣�
����

 Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д���9�֣�1 Lij�����Һ�����ܺ��е��������±���
| ���ܴ������е������� | H+ NH4+ Al3+ K+ |
| ���ܴ������е������� | Cl- Br- I‑ ClO‑ AlO2- |
(1)������Һ����μ���NaOH��Һ���ʵ����ȣ�������������������ʵ����������NaOH��Һ�������
���Ĺ�ϵ����ͼ��ʾ�������Һ��ȷ�����е�������_______________��
����ȷ���Ƿ��е���������__________��Ҫȷ������ڿɲ�������ʵ����________��
��2������⣬����Һ�к��д�����Cl�� ��Br����I��������1 L�û����Һ��ͨ�롪������Cl2����Һ��Cl����Br����I�������ʵ�����ͨ��Cl2�������״�����Ĺ�ϵ���±���ʾ��������ش��������⣺
| Cl2���������״���� | 2.8L | 5.6 L | 11.2 L |
|
| 1.25mol | 1.5 mol | 2 mol |
|
| 1.5 mol | 1.4 mol | 0.9 mol |
|
|
| 0 | 0 |
�ٵ�ͨ��Cl2�����Ϊ2.8 Lʱ����Һ�з�����Ӧ�����ӷ���ʽΪ___ ��
��ԭ��Һ��Cl����Br����I�������ʵ���Ũ��֮��Ϊ_____________��
1 Lij�����Һ�����ܺ��е��������±���
| ���ܴ������е������� | H+ NH4+ Al3+ K+ |
| ���ܴ������е������� | Cl- Br- I‑ ClO‑ AlO2- |
��1��������Һ����μ���NaOH��Һ���ʵ����ȣ�������������������ʵ������������NaOH��Һ�������
���Ĺ�ϵ����ͼ��ʾ��
�����Һ��ȷ�����е�������____________________��
����ȷ���Ƿ��е���������____________________��
Ҫȷ������ڿɲ�������ʵ����____________________��
�϶������ڵ���������____________________��
��2������⣬����Һ�к��д�����Cl�� ��Br����I��������1 L�û����Һ��ͨ��һ������Cl2����Һ��Cl����Br����I�������ʵ�����ͨ��Cl2�������״�����Ĺ�ϵ���±���ʾ��������ش��������⣺
| Cl2���������״���� | 2��8L | 5��6 L | 11��2 L |
|
| 1��25mol | 1��5 mol | 2 mol |
|
| 1��5 mol | 1��4 mol | 0��9 mol |
|
|
| 0 | 0 |
�ٵ�ͨ��Cl2�����Ϊ2��8Lʱ����Һ�з�����Ӧ�����ӷ���ʽΪ____________________��
��ԭ��Һ��Cl����Br����I�������ʵ���Ũ��֮��Ϊ______________________________��
��12�֣�1 Lij�����Һ�����ܺ��е��������±���
|
���ܴ������е������� |
H+ NH4+ Al3+ K+ |
|
���ܴ������е������� |
Cl- Br- I‑ ClO‑ AlO2- |
(1)������Һ����μ���NaOH��Һ���ʵ����ȣ�������������������ʵ������������NaOH��Һ����������Ĺ�ϵ����ͼ��ʾ��
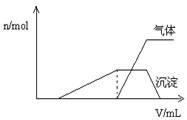
�����Һ��ȷ�����е������� ��
����ȷ���Ƿ��е��������� ��
Ҫȷ������ڿɲ�������ʵ���� ��
�϶������ڵ��������� ��
��2������⣬����Һ�к��д�����Cl�� ��Br����I��������1 L�û����Һ��ͨ��һ������Cl2����Һ��Cl����Br����I�������ʵ�����ͨ��Cl2�������״�����Ĺ�ϵ���±���ʾ��������ش��������⣺
|
Cl2���������״���� |
2��8L |
5��6 L |
11��2 L |
|
��Cl���� |
1��25mol |
1��5 mol |
2 mol |
|
��Br���� |
1��5 mol |
1��4 mol |
0��9 mol |
|
��I���� |
mol |
0 |
0 |
�ٵ�ͨ��Cl2�����Ϊ2��8 Lʱ����Һ�з�����Ӧ�����ӷ���ʽΪ ��
��ԭ��Һ��Cl����Br����I�������ʵ���Ũ��֮��Ϊ ��
��12�֣�1 Lij�����Һ�����ܺ��е��������±���
|
���ܴ������е������� |
H+ NH4+ Al3+ K+ |
|
���ܴ������е������� |
Cl- Br- I‑ ClO‑ AlO2- |
(1)������Һ����μ���NaOH��Һ���ʵ����ȣ����� ��������������ʵ����� �������NaOH��Һ�������
�������NaOH��Һ������� ���Ĺ�ϵ����ͼ��ʾ��
���Ĺ�ϵ����ͼ��ʾ��
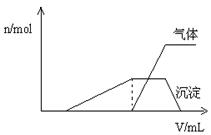
�����Һ��ȷ�����е�������____ ___ __��
����ȷ���Ƿ��е���������_______ ___��
Ҫȷ������ڿɲ�������ʵ����______ _��
�϶������ڵ���������________ ___��
��2������⣬����Һ�к��д�����Cl�� ��Br����I��������1 L�û����Һ��ͨ��һ������Cl2����Һ��Cl����Br����I�������ʵ�����ͨ��Cl2�������״�����Ĺ�ϵ���±���ʾ��������ش��������⣺
|
Cl2���������״���� |
2��8L |
5��6 L |
11��2 L |
|
|
1��25mol |
1��5 mol |
2 mol |
|
|
1��5 mol |
1��4 mol |
0��9 mol |
|
|
|
0 |
0 |
�ٵ�ͨ��Cl2�����Ϊ2��8 Lʱ����Һ�з�����Ӧ�����ӷ���ʽΪ__ _��
��ԭ��Һ��Cl����Br����I�������ʵ���Ũ��֮��Ϊ_____ ________��
 mol
mol