��Ŀ����
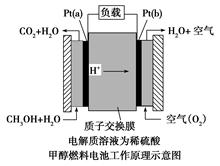
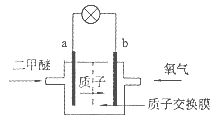
������������Դ���������й㷺��;������(Ni)�������缫���ŨNaOH��Һ�Ʊ���������(Na2FeO4)��װ����ͼ��ʾ������˵����ȷ����
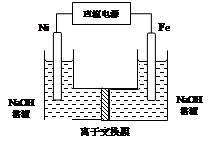

A�������������缫��ӦΪFe��2e����2OH�� Fe(OH)2 Fe(OH)2 |
| B�����һ��ʱ������缫������Һ��pH��С |
| C�������ӽ���ĤΪ�����ӽ���Ĥ����������������Һ�к���FeO42�� |
| D��ÿ�Ƶ�1mol Na2FeO4�������Ͽ��Բ���67.2L���� |
C
���������������Ni���������缫���ŨNaOH��Һ�Ʊ��������ƣ�Na2FeO4������ʧ�������ɸ������ƣ�������������������������������ʧ���������������ӣ��������Ӻ����������ӷ�Ӧ�������������������Ե缫��ӦʽΪFe-2e��+2OH���TFe(OH)2����A��ȷ�����缫�������ӷŵ�����������������Ũ�ȼ�С��������Һ��pH����B���������ӽ���ĤΪ�����ӽ���Ĥ���������������ƶ�������������Ҳ���Һ�к���FeO42������C�����¶Ⱥ�ѹǿδ֪���������������������������D����ѡA��

��ϰ��ϵ�д�
 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�����Ŀ